Bianca Maria Maglia OrlandiI; Omar Asdrúbal Vilca MejiaI; Evelinda Marramon TrindadeIII; Fabio B. JateneI
DOI: 10.21470/1678-9741-2022-0261
ABSTRACT
Introduction: Deep sternal wound infections (DSWI) are so serious and costly that hospital services continue to strive to control and prevent these outcomes. Microcosting is the more accurate approach in economic healthcare evaluation, but there are no studies in this field applying this method to compare DSWI after isolated coronary artery bypass grafting (CABG). This study aims to evaluate the incremental risk-adjusted costs of DSWI on isolated CABG.BMI = Body mass index
CABG = Coronary artery bypass grafting
CCS = Canadian Cardiovascular Society
CI = Confidence interval
COPD = Chronic obstructive pulmonary disease
CSQI = Cardiac Surgery Quality Initiatives
DRG = Diagnosis-related groups
DSWI = Deep sternal wound infections
FAPESP = Fundação de Amparo à Pesquisa do Estado de São Paulo
HbA1C = Hemoglobin A1C
ICD-10 = International Classification of Diseases, Tenth Revision
ICU = Intensive care unit
LOS = Length of stay
NCSP = NOMESCO Classification of Surgical Procedures
NYHA = New York Heart Association
PSM = Propensity score matching
REPLICCAR = Registro Paulista de Cirurgia Cardiovascular
SD = Standard deviation
STS ACSD = Society of Thoracic Surgeons Adult Cardiac Surgery Database
INTRODUCTION
Deep sternal wound infections (DSWI) as a result of open-heart surgery are so serious and costly that hospital services continue to strive to control and prevent these outcomes[1,2]. The prevalence of DSWI in coronary artery bypass grafting (CABG) patients varies between 1% and 4% worldwide[3,4]. And multiple risk factors are associated with infections in cardiac surgery, such as female sex, age, diabetes, obesity, renal failure, smoking, steroid use, and chronic obstructive pulmonary disease (COPD)[1,4,5].
In Brazil, a study reported the total cost of CABG per patient of US$7,992.55[6]. Prior estimates of the cost of hospitalizations after surgical infections vary widely across hospitals, states, and regions, and range from US$24,000 to US$58,000[7-9].
Quality improvement practices were first implemented by Ernest Codman, who migrated from the technology industry to clinical practice and collaborated to improve outcomes, even with high-complexity procedures involved[10]. Throughout the study of the Registro Paulista de Cirurgia Cardiovascular (REPLICCAR), our team participates in the Cardiac Surgery Quality Program to identify opportunities for quality improvement in cases in which high-cost and resource-intensive frequently preventable outcomes might occur. Quality interventions do not necessarily imply increased hospital costs, as it focuses on the optimization of an existing organizational process. Some examples are medical care focuses on patients, protocols based on the best available evidence, decisions made by a multidisciplinary team, real-time data to show quality improvement protocols, benefits of interventions and their impact on patients, and education leading to positive changes[10,11].
There is a need to establish appropriate priorities between patients’ groups with an effective selection for treatment within particular characteristics, based on the risk of complications and chance of survival, rehabilitation, and acceptable quality of life. Risk scores have become an important tool in patient assessment, including factors such as age, the severity of heart disease, and co-morbidity in the type of cardiac procedure. However, most scoring systems are used to predict mortality, and further refinement to specific morbidity risk scores is necessary to predict both outcome and hospital costs[11].
Microcosting is the more accurate method to describe economic evaluation in healthcare. It can provide the most precise approach of deriving interventional costs because it involves direct enumeration and costing of each interventional input, such as nurse or pharmacist time for the procedure, and capital inputs, such as facilities space. The process includes three stages: (1) identification of all resources involved in the provision of care (e.g., human resources, consumables/materials); (2) accurate measurement of each resource (e.g., time and motion studies); (3) valuation of the resources used[12]. Only a few studies reported this method in cardiac surgery[11,13-16], none of them used the microcosting approach to estimate hospital costs for DSWI as a severe and costly complication in postoperative patients of cardiac surgery.
In that view, DSWI and mediastinitis represent a preventable outcome with a resource-intensive environment. The purpose of our study was to estimate the cost of DSWI and mediastinitis in a sample of isolated CABG patients from a referenced cardiac hospital in São Paulo, Brazil.
METHODS
This is a retrospective observational cohort study using a single center for the microcosting analysis of patients with DSWI and without complications after isolated CABG as the first cardiac procedure. Data were obtained from the REPLICCAR II database, which was a multicentric cohort study performed by voluntary participant hospitals between August 2017 and June 2019. The variables included in REPLICCAR II were defined using the Society of Thoracic Surgeons Adult Cardiac Surgery Database (STS ACSD) collection tool (version 2.9 - 2017). Approximately 760 variables were collected preoperatively, intraoperatively, and postoperatively, and included risk factors, clinical and laboratory characteristics, and complications of surgery. The data were collected using a secure web application for building and managing online surveys and databases, the REDCap platform (Research Electronic Data Capture, https://www.project-redcap.org/).
The Comissão de Ética para Análise de Projetos de Pesquisa (ethical committee board) approved the study under the protocol number CAPPesq: 2.507.078 and received funding from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (CNPq PPSUS FAPESP 2016/15163-0); patient consent was not required.
Criteria and Definitions
DSWI were classified according to the Centers for Disease and Control National Healthcare Safety Network (or CDC NHSN) criteria and definitions[17]. Profound infections (involving fascia, muscle layers, and/or deep soft tissue) and organs/spaces infections (mediastinitis/osteomyelitis) were included. Superficial infections were excluded due to discrepancies in treatment compared to deep infections.
During the REPLICCAR II study, the surgical surveillance system followed patients until 30 days after surgery, where they received a call after discharge asking about their recovery. Thus, patients that persisted in the hospital and developed infections were included in this analysis. The groups were divided into control, deep infections, and, separately, mediastinitis/osteomyelitis.
Adjusted Costs
To conduct our analysis, the values were corrected by the inflation costs variation on the General Market Price Index of the Fundação Getúlio Vargas adopting the study period for calculation (August 2017 to July 2019). Also, the dollar quotation variation on the period was considered to estimate the costs in American dollars. The data is available online for public consultation (http://ipeadata.gov.br/Default.aspx).
Missing Data
The REPLICCAR II database includes a total of nine eligible centers in São Paulo (Brazil), with data of patients that underwent CABG from 2017 to 2019. The missing data in the database was mostly related to clinical characteristics, missing completely at random, and it was < 30%. Multiple imputations by chained equations (or MICE) were performed in R Studio software with 10 imputations (n=4085 observations, P=161 variables). After imputation, data from a single center was captured to guarantee a homogenous sample comparison between infected and non-infected patients using propensity score matching (PSM).
Propensity Score Matching
PSM considered matching nearest neighbor. The predictors included patients with infections who died until 30 days of follow-up. Dependent variables were preoperative in-hospital duration, gender, body mass index, prior family history of coronary disease, previous myocardial infarction, hypertension, peripheral artery disease, renal failure, dyslipidemia, insulin dependence, previous percutaneous intervention, COPD, surgical status, angina Canadian Cardiovascular Society classification, and intraoperative blood transfusion. The treatment group included patients with wound interventions after surgery.
Microcosting Analysis
To include the costs of each component and apply a microcosting evaluation, we took additional data from the hospital system (Si3) to get detailed information related to all resources available, such as additional hospital stays, utilities and medications, re-interventions, clinical, laboratory and image tests, bandage, etc.
Statistical analyses were conducted using STATA 16.1 (StataCorp, College Station, Texas, United States of America) software package. Costs and length of stay (LOS) were described with average and 95% confidence intervals (CI). Linear regression was performed to evaluate factors related to increased costs between groups. Analysis of variance was used to compare differences between groups, and P<0.05 was considered significant.
RESULTS
A total of 1,120 CABGs were performed in the hospital between 2017 and 2019. The DSWI prevalence during the period was 4.7% (n=53), and prevalence of mediastinitis/osteomyelitis was 1.4% (n=16). The STS risk score for mortality was on average 1.14% (standard deviation [SD]=0.9) in the DSWI group and 1.07% (SD=0.8) for patients without infections; and the STS model for DSWI (including mediastinitis) was 0.19% in average (SD=0.07) on the infected and 0.12% (SD=0.8) on non-infected patients. Seven patients died within 30 days (10.7%) - one in the same hospitalization (in-hospital mortality) and another six were re-admitted until 30 days after CABG (five with mediastinitis and one with deep infection). After PSM, 66 patients were allocated between groups. Sample characteristics after matching groups are described in Table 1.
| Characteristics | Control (n=30) n (%) |
DSWI (n=26) n (%) |
Mediastinitis (n=10) n (%) |
P-value |
|---|---|---|---|---|
| Age, average ± SD | 62.1±10.2 | 62.4±9.8 | 66.8±5.9 | 0.379 |
| Female sex | 14 (40) | 16 (45.7) | 5 (14.3) | 0.527 |
| Diabetes | 20 (44.4) | 19 (42.2) | 6 (13.3) | 0.728 |
| Insulin-dependent | 2 (20) | 5 (50) | 3 (30) | 0.136 |
| Hypertension | 24 (43.6) | 22 (40) | 9 (16.4) | 0.823 |
| Dyslipidemia | 19 (48.7) | 13 (33.3) | 7 (18.0) | 0.521 |
| BMI > 30 (Kg/cm2) | 10 (45.5) | 9 (40.9) | 3 (13.6) | 1.000 |
| Chronic obstructive pulmonary disease | 1 (50) | 0 (0) | 1 (50) | 0.282 |
| NYHA ≥ III | 5 (33.3) | 5 (33.3) | 5 (33.3) | 0.080 |
| Angina CCS 4 | 6 (50) | 5 (41.7) | 1 (8.3) | 0.833 |
| Peripheral artery disease | 4 (50) | 4 (50) | 0 (0) | 0.607 |
| Previous myocardial infarction | 9 (33.3) | 14 (51.9) | 4 (14.8) | 0.212 |
| Elective status | 15 (50.0) | 11 (42.3) | 6 (60.0) | 0.620 |
| Preoperative ICU admission | 14 (40) | 16 (45.7) | 5 (14.3) | - |
| Days in hospital before surgery | 5.6 | 5.8 | 4 | 0.698 |
| Preoperative exams | ||||
| HbA1c (%), average ± SD | 6.4±1.1 | 7.6±2.1 | 7.4±1.3 | 0.024 |
| Glucose (mg/dL), average ± SD | 136±46 | 166±80 | 143±63 | 0.226 |
| Creatinine (mg/dL), average ± SD | 1.0±0.2 | 1.2±1.1 | 1.8±1.6 | 0.071 |
Chi-square or Fisher’s exact test or analysis of variance BMI=body mass index; CABG=coronary artery bypass grafting; CCS=Canadian Cardiovascular Society; DSWI=deep sternal wound infections; HbA1C=hemoglobin A1C; ICU=intensive care unit; NYHA=New York Heart Association; SD=standard deviation
Figure 1 describes the hospitalization total costs between groups. As expected, deep infection increased the costs by 152%, and mediastinitis increased it by 188%. The mean cost for the control group was $6,863 (SD=4,615); for deep infections, it was $17,329 (SD=8,471); and for mediastinitis it was $19,805 (SD=13,383).
Figure 2 describes the microcosting analysis with the average financial impact of infections on costs for each hospital department involved with the cardiac surgery process. Hospital stays, special materials, laboratory and image exams, medications and nutrition, and multidisciplinary labor were the costliest services for infected patients and were statistically different compared to the control group. However, the cost driver identified by the multiple linear regression was the hospital stay (b=0.0001; adj R2=0.48; 95% CI .00006-.0001; P=0.000).
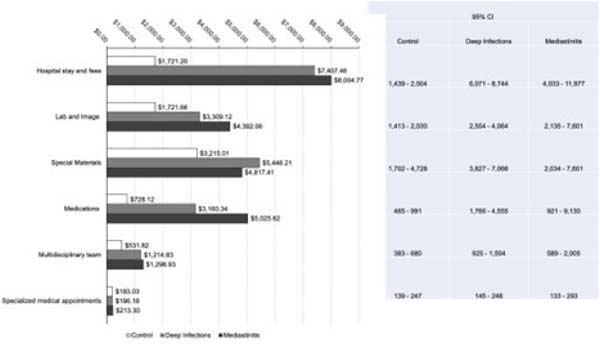
Considering time in intensive care unit (ICU), 19.2% and 40% of patients with deep infections and mediastinitis were readmitted for intensive care. The mean LOS in ICU after readmission was 13 days in the deep infections group and 21 days in the mediastinitis group. The groups were statistically different in total postoperative duration, where the control group total LOS was 9.5 days, the DSWI was 37 days, and the mediastinitis was 59 days (P=0.000).
DISCUSSION
Little information is available on the final impact of infections after major cardiac surgery. DSWI results in significant patient morbidity and consumes considerable resources and hospital LOS[18,19]. The main purpose of this study was to provide an accurate description of how much infections (DSWI and mediastinitis) increase costs related to open surgical treatment in CABG patients. It should come as no surprise that there is a strong positive correlation between LOS and hospital costs. What is of interest is the relative importance of each complication to the cost structure of isolated CABG patients and the hospital’s ability to create policymakers to predict and manage these circumstances[9].
Still, previous studies confirm our findings and reinforce the need for preventive methods[18]. Graf et al. report that the main proportion of costs related to DSWI concerns to ward care, additional interventions, and prolonged ICU care[20]. Besides the prolonged hospital stay, mortality and morbidity rates during treatment are high. Gib et al. report that using preconditioning wounds, such as vacuum therapy, could reduce mortality by 22% compared to time-only procedures[21].
On the other hand, Brandt et al.[22] published a structured literature review with data from 14 countries, including Brazil. The paper explores the burden of surgical infections after CABG. However, infection rates varied considerably between settings, with infections occurring in 2.8% (the United Kingdom) to 10.4% (the Netherlands) of CABG procedures, while the costs per surgical infection varied between $8,172 (Brazil) to $54,180 (Japan). Important limitations of this analysis include uncertainty about the surveillance methods, criteria and definitions, and superficial infections.
Economic evaluation studies in surgery frequently use “top-down” or “gross-costing” approaches and, usually, are based on healthcare resource groups, which can be used to estimate the average cost per inpatient episode for groups of surgical procedures[11]. The diagnosis-related groups (DRG) were a framework created for monitoring hospital activity and efficiency and thus to control the increasing hospital costs better. The unique DRG to which any procedure is assigned is based on disease, comorbidities, and complications, as recorded by the International Classification of Diseases, Tenth Revision (ICD-10). Treatment codes are given by the NOMESCO Classification of Surgical Procedures (NCSP); the national DRG code is automatically calculated by a computer algorithm introducing the ICD-10 codes and NCSP codes. The algorithm is constructed to allocate the most complicated patients to the lowest DRG code number[6]. However, there are several limitations related to these methods, such as the need to compare two different surgical procedures within the same group or evaluate a modification/actualization to an existing process[12].
Brazilian economic and health systems databases are challenging to manage costs or quality because the ICD-10 is biased and does not represent clinical characteristics. Data collection is very scarce, and most observations are summaries per region, so individual observations aren’t possible using the National Database. In this study, we opted to use the REPLICCAR II database due to all limitations in Brazilian sources.
Prediction models designed for specific outcomes consistent with real population parameters may provide more accurate information about patients and hospital resources. Developers of performance measures will also be expected to promote their measures to health systems and payers. Another tough work would be to best educate consumers about why the measures are important to increase quality. Fortunately, such measure-promotion efforts will be synergistic with registry-promotion activities. Through advocacy for wider measure adoption, models can simultaneously promote the use of their performance measures and the registries that report those measures, thereby furthering the goals of patient-centered care[22].
The STS models, for example, are widely used by Cardiac Surgery Quality Initiatives (CSQI) programs, such as the Virginia CSQI and the American Association for Cardiac Surgery[23]. The Virginia CSQI evaluates adherence to clinical and process metrics derived from performance measures from the STS ACSD. This voluntary consortium of 17 hospitals and 13 cardiac surgical practices in Virginia (United States of America) identified quality improvement opportunities. It tracked patient outcomes but also found options for cost containment, such as improved patient outcomes and decreased resource utilization[24,25,26].
In 2018, the Centers for Medicare & Medicaid Services announced that the bundled payments for care improvement advanced. The bundled payments, also described as episode payment models, are designed to move toward value-based care by incentivizing providers to go above the target price for an episode, including those that arise from complications and hospital readmissions. The idea is to support quality programs that invest in practice innovation and care redesign to better coordinate and reduce expenditures while improving the quality of care[27,28].
In addition, Brescia et al.[27] (2020) reported that assessing tradeoffs between spending and quality is essential for success in bundled reimbursement models. Although the authors didn’t evaluate the tradeoffs, they made a retrospective observation of 33 nonfederal hospitals in Michigan (United States of America) and identified determinants of variability between hospitals.
Implementing quality programs may represent the key to success for continuous improvement results.
Limitations
Several limitations can be related to this observational, retrospective design, which cannot account for all potential confounding variables in this situation. The study criteria included only sternal infections, and the follow-up and readmissions data were considered. However, in our scenario, this study provides an estimated cost for infections in isolated CABG. It allows us to use clinical data in healthcare management to provide excellent quality based on knowledge. We must note that we based our data on a single Brazilian institution, and costs may not be generalized for other facilities and country regions.
CONCLUSION
In summary, our results demonstrate the incremental costs of a detailed microcosting evaluation of infections on CABG patients in São Paulo, Brazil. Hospital stay was an important cost driver identified, demonstrating the importance of evaluating patients’ characteristics and managing risks for a faster, safer, and more effective discharge.
Data Availability
The data generated during the current study are not publicly available due to ethical restrictions; patients did not consent to their deidentified data being publicly shared, but these data are available on reasonable request to the Scientific Committee Director Renata do Val (renata.doval@incor.usp.br; https://www.incor.usp.br/sites/incor2013/index.php/16-pesquisa/comissao-cientifica/158-fale-conosco).
REFERENCES
1. Tiveron MG, Fiorelli AI, Mota EM, Mejia OA, Brandão CM, Dallan LA, et al. Preoperative risk factors for mediastinitis after cardiac surgery: analysis of 2768 patients. Rev Bras Cir Cardiovasc. 2012;27(2):203-10. doi:10.5935/1678-9741.20120035. [MedLine]
2. Nelson RM, Dries DJ. The economic implications of infection in cardiac surgery. Ann Thorac Surg. 1986;42(3):240-6. doi:10.1016/s0003-4975(10)62726-9. [MedLine]
3. Nieminen VJ, Jääskeläinen IH, Eklund AM, Murto ES, Mattila KJ, Juvonen TS, et al. The characteristics of postoperative mediastinitis during the changing phases of cardiac surgery. Ann Thorac Surg. 2021;112(4):1250-6. doi:10.1016/j.athoracsur.2020.10.029. [MedLine]
4. Vun S, Newland R, Bennetts J, Baker R. Accuracy of Fowler’s score in predicting deep sternal wound infection among cardiac surgery patients. Heart Lung Circ. 2016;25(8):e116. doi:10.1016/j.hlc.2015.12.086.
5. Vos RJ, Van Putte BP, Kloppenburg GTL. Prevention of deep sternal wound infection in cardiac surgery: a literature review. J Hosp Infect. 2018;100(4):411-20. doi:10.1016/j.jhin.2018.05.026.
6. Silva GSD, Colósimo FC, Sousa AG, Piotto RF, Castilho V. Coronary artery bypass graft surgery cost coverage by the Brazilian unified health system (SUS). Braz J Cardiovasc Surg. 2017;32(4):253-9. doi:10.21470/1678-9741-2016-0069.
7. Taylor GJ, Mikell FL, Moses HW, Dove JT, Katholi RE, Malik SA, et al. Determinants of hospital charges for coronary artery bypass surgery: the economic consequences of postoperative complications. Am J Cardiol. 1990;65(5):309-13. doi:10.1016/0002-9149(90)90293-a.
8. Mauldin PD, Weintraub WS, Becker ER. Predicting hospital costs for first-time coronary artery bypass grafting from preoperative and postoperative variables. Am J Cardiol. 1994;74(8):772-5. doi:10.1016/0002-9149(94)90432-4.
9. LaPar DJ, Crosby IK, Rich JB, Fonner E Jr, Kron IL, Ailawadi G, et al. A contemporary cost analysis of postoperative morbidity after coronary artery bypass grafting with and without concomitant aortic valve replacement to improve patient quality and cost-effective care. Ann Thorac Surg. 2013;96(5):1621-7. doi:10.1016/j.athoracsur.2013.05.050.
10. Mejía OA, Lisboa LA, Jatene FB. Continuous quality improvement programme in cardiovascular surgery: the Latin American perspective. Eur J Cardiothorac Surg. 2016;50(1):4-5. doi:10.1093/ejcts/ezw087.
11. Mishra V, Geiran O, Krohg-Sørensen K, Andresen S. Thoracic aortic aneurysm repair. Direct hospital cost and diagnosis related group reimbursement. Scand Cardiovasc J. 2008;42(1):77-84. doi:10.1080/14017430701716814.
12. Potter S, Davies C, Davies G, Rice C, Hollingworth W. The use of microcosting in economic analyses of surgical interventions: a systematic review. Health Econ Rev. 2020;10(1):3. doi:10.1186/s13561-020-0260-8.
13. Coyan G, Wei LM, Althouse A, Roberts HG, Schauble D, Murashita T, et al. Robotic mitral valve operations by experienced surgeons are cost-neutral and durable at 1 year. J Thorac Cardiovasc Surg. 2018;156(3):1040-7. doi:10.1016/j.jtcvs.2018.03.147.
14. Fanourgiakis J, Simantirakis E, Maniadakis N, Kourlaba G, Kanoupakis E, Chrysostomakis S, et al. Cost-of-illness study of patients subjected to cardiac rhythm management devices implantation: results from a single tertiary centre. Europace. 2013;15(3):366-75. doi:10.1093/europace/eus363.
15. Kanters TA, Wolff C, Boyson D, Kouakam C, Dinh T, Hakkaart L, et al. Cost comparison of two implantable cardiac monitors in two different settings: reveal XT in a catheterization laboratory vs. reveal LINQ in a procedure room. Europace. 2016;18(6):919-24. doi:10.1093/europace/euv217.
16. Lattimer CR, Azzam M, Kalodiki E, Shawish E, Trueman P, Geroulakos G. Cost and effectiveness of laser with phlebectomies compared with foam sclerotherapy in superficial venous insufficiency. Early results of a randomised controlled trial. Eur J Vasc Endovasc Surg. 2012;43(5):594-600. doi:10.1016/j.ejvs.2012.01.032.
17. Centers for Disease and Control. CDC/NHSN Surveillance Definitions for Specific Types of Infections: Surveillance Definitions. CDC. 2020;(January):1–36.
18. Speir AM, Kasirajan V, Barnett SD, Fonner E Jr. Additive costs of postoperative complications for isolated coronary artery bypass grafting patients in Virginia. Ann Thorac Surg. 2009;88(1):40-5; discussion 45-6. doi:10.1016/j.athoracsur.2009.03.076.
19. Ferreira GB, Donadello JCS, Mulinari LA. Healthcare-associated infections in a cardiac surgery service in Brazil. Braz J Cardiovasc Surg. 2020;35(5):614- 8. doi:10.21470/1678-9741-2019-0284.
20. Graf K, Ott E, Vonberg RP, Kuehn C, Haverich A, Chaberny IF. Economic aspects of deep sternal wound infections. Eur J Cardiothorac Surg. 2010;37(4):893-6. doi:10.1016/j.ejcts.2009.10.005.
21. Gib MC, Alvarez JS, Wender OCB. Mediastinitis: mortality rate comparing single-stage surgical approach and preconditioning of wound. Braz J Cardiovasc Surg. 2013;28(2):200–7. doi: 10.5935/1678-9741.20130029.
22. Brandt D, Blüher M, Lankiewicz J, Mallow PJ, Saunders R. Sternal-wound infections following coronary artery bypass graft: could implementing value-based purchasing be beneficial? J Health Econ Outcomes Res. 2020;7(2):130-8. doi:10.36469/jheor.2020.13687.
23. WRITING COMMITTEE MEMBERS; Bhatt DL, Drozda JP Jr, Shahian DM, Chan PS, Fonarow GC, et al. ACC/AHA/STS statement on the future of registries and the performance measurement enterprise: a report of the American college of cardiology/American heart association task force on performance measures and the society of thoracic surgeons. Circ Cardiovasc Qual Outcomes. 2015;8(6):634-48. doi:10.1161/HCQ.0000000000000013.
24. Blackstone EH, Swain J, McCardle K, Adams DH; Governance Committee, American Association for Thoracic Surgery Quality Assessment Program. A comprehensive American association for thoracic surgery quality program for the 21st century. J Thorac Cardiovasc Surg. 2019;158(4):1120- 6. doi:10.1016/j.jtcvs.2019.07.017.
25. LaPar DJ, Crosby IK, Ailawadi G, Ad N, Choi E, Spiess BD, et al. Blood product conservation is associated with improved outcomes and reduced costs after cardiac surgery. J Thorac Cardiovasc Surg. 2013;145(3):796-803; discussion 803-4. doi:10.1016/j.jtcvs.2012.12.041.
26. Prager RL, Armenti FR, Bassett JS, Bell GF, Drake D, Hanson EC, et al. Cardiac surgeons and the quality movement: the Michigan experience. Semin Thorac Cardiovasc Surg. 2009;21(1):20-7. doi:10.1053/j.semtcvs.2009.03.008.
27. Brescia AA, Vu JV, He C, Li J, Harrington SD, Thompson MP, et al. Determinants of value in coronary artery bypass grafting. Circ Cardiovasc Qual Outcomes. 2020;13(11):e006374. doi:10.1161/CIRCOUTCOMES.119.006374.
28. Farmer SA, Darling ML, George M, Casale PN, Hagan E, McClellan MB. Existing and emerging payment and delivery reforms in cardiology. JAMA Cardiol. 2017;2(2):210-7. Erratum in: JAMA Cardiol. 2019;4(1):84. doi:10.1001/jamacardio.2016.3965.
Authors’Roles & Responsibilities
BMMO= Substantial contributions to the acquisition and analysis of data for the work; drafting the work and revising it; final approval of the version to be published
OAVM= Substantial contributions to the conception of the work; drafting the work and revising it; final approval of the version to be published
EMT= Substantial contributions to the acquisition and analysis of data for the work; revising the work; final approval of the version to be published
FBJ= Substantial contributions to conception of the work; revising the work; final approval of the version to be published
Article receive on Monday, July 11, 2022
Article accepted on Thursday, February 23, 2023
 All scientific articles published at bjcvs.org are licensed under a Creative Commons license
All scientific articles published at bjcvs.org are licensed under a Creative Commons license