INTRODUCTION
The basic principle that guided the development of techniques of myocardial preservation was the ischemia/reperfusion lesion. The repair of anomalies and disorders of the heart by itself is not enough to provide energy substrates and electrolytes to the myocardium, especially in case of prolonged periods of ischemia [1-5].
The thoroughly prevention of both ischemia and reperfusion lesions during heart surgery using cardiopulmonary bypass (CPB) has been restricted to myocardial protection. However, it is theoretically known that the primary function of the CPB would consider both metabolic and energy demands of the heart, as well as that of the lungs [4-6].
In heart surgery with CPB, the use of aortic clamping technique in combination with cardioplegia prevents the right ventricle to maintain perfusion of the lungs. Similarly, in operations with CPB support, in which the heart is kept beating without aortic clamping, the perfusion of the lungs is also jeopardized, once there is a drainage partial or total of blood volume from the right chambers, consequently, limiting the perfusion flow of the pulmonary trunk. To all this must also be added, the observation made by Schlensak et al. [7] that the bronchial arterial flow turns up to be very low and variable during CPB because the use of non-pulsatile flow.
It should keep in mind that there is a close relationship between the right ventricle and the pulmonary vasculature, comprising the pulmonary trunk and its peripheral capillary extensions. In the light of this, the right ventricular systole and, therefore, the work performed by the right ventricle depend on hemodynamic parameters, such as MPAP and PVR.
By a regime of high levels of these cited parameters, signs of overload and right ventricular dysfunction can be identified through clinical, electrocardiographic, and echocardiographic features. One of the main clinical features of right ventricular overload would be the increased tissue BNP synthesis, once this peptide has been described by many authors as one of the most relevant indirect markers of ventricular overload [80-10].
Once the lungs are perfused during CPB with perfusion pressure controlled, the deleterious effects caused by the CPB regarding the significant increase in MPAP and PVR can be attenuated. Thus, the right ventricle performance could be optimized and occasional signs of overload would certainly be avoided. Thus, one can postulate that the pulmonary perfusion with perfusion pressure controlled tends not to cause right ventricular overload in the post-CPB period. Thereafter, BNP values expressed by the right ventricular myocardium would be not significantly elevated [7,11].
On an ad hoc basis, Nagaya et al. [11] demonstrated that in the postoperative period of pulmonary thromboendarterectomy, besides the reduced levels of MPAP and PVR, there is also a significant reduction of BNP synthesis of the right ventricle. The authors point out that there is a correlation between the decreased MPAP and PVR levels, and the reduced BNP level by the right ventricle. Therefore, one can understand that the inadequate perfusion of the lungs could have a negative impact both of mechanical and inflammatory nature in the right ventricle myocardium.
In light of all this information, this study aims to determine whether perfusion of the pulmonary trunk can modify the BNP tissue expression through the right ventricle in surgeries with CPB.
METODS
Animals
After approval of the research by the Local Ethics Animal Research Committee, an experimental study was conducted at the laboratory of experimental operative technique and surgery in the Federal University of São Paulo. The handling of these animals followed the guidelines of the "Guide for the Care and Use of Laboratory Animals" and the ethical principles for animal experimentation of the Brazilian College of Animal Experimentation. In the present study, it was used 32 male porcine with a mean age of 2.7 months, mean weight of 20.4 kg, average length of 70.3 cm, all belonging to the lineage Pernalan C-56. To perform this experimental research, we have chosen the porcine because they present from the cardiac and pulmonary standpoint, due to their anatomical and physiological similarities with the human beings. The animals, which presented no clinical or laboratory signs of infection in any site have been considered eligible for inclusion in the study.
Study design
In the present research, labels numbered from 1 through 32 were placed in a sealed box. For each animal, prior to the surgical procedure and after its separation from the others, a label was taken at random from the box. The animals that were labeled with the numbers 1-4, 5-10, and 11-16, respectively belong to subgroups IA, IB and IC. The animals that were labelled with the numbers 17-20, 21-26, and 27-32, respectively belong to subgroups IIA, IIB and IIC.
The operative procedure was performed immediately after the animal was randomized. The random process was performed again only at the end of each operative procedure.
Thus, the animals were randomly divided into two main groups according to the strategy of CPB - Group I (n = 16 - cardioplegia) and Group II (n = 16 - beating heart). Each of the main groups were also randomly subdivided into three subgroups according to the strategy of the pulmonary perfusion - Group A (n = 4) control - without pulmonary perfusion (IA / IIA); group B ( n = 6) - perfusion with the pulmonary arterial blood (IB / IIB); and subgroup C (n = 6) - perfusion with the pulmonary venous blood (IC / IIC).
Anesthesia and preoperative procedure
The animals received as pre-aesthetic drugs, acepromazine (0.1 mg / kg) and ketamine (10 mg / kg), both intramuscularly. After 30 minutes, an anesthetic induction through an intravenous injection of sodium pentobarbital at a dosage of 12.5 mg / kg, in the ear right marginal vein was performed. The animals were kept on mechanical ventilation, with a respiratory frequency of approximately 14 breaths per minute and a tidal volume of 10 ml / kg. Anesthesia was maintained with fentanyl (0.01 mg/kg intravenous) and thiopental (1 g intravenous) at every 20 minutes. An 8 Fr polyethylene catheter was introduced into the right internal carotid artery under anesthesia and by dissection and phlebotomy for measurement of mean arterial pressure. A 7-gauge Fr Swan-Ganz catheter (93A-131H-7F, Edwards, Baxter Edwards Critical Care, Irvine, CA) was inserted into the right jugular vein by dissection and phlebotomy. Its tip was positioned in the pulmonary artery guided by the pressure traces. These catheters were connected to individual pressure transducers and a polygraph model Viridia 24C (Hewlett-Packard Corp., Andover, MS, USA) to measure hemodynamic parameters, such as mean pulmonary artery pressure. A third polyethylene catheter (8 Fr) was inserted into the external jugular vein by dissection and phlebotomy. It was used as a central venous access.
CPB and Pulmonary Perfusion
In the animals in the cardioplegia group I, after performing systemic therapeutic administration of heparin (4 mg / kg), the CPB support was performed by cannulation of the aorta and bicaval cannulation. Two cardioplegia solutions were performed, the first just after aortic clamping and the second after a 15-minute interval. Once the total CPB was established, the mechanical ventilation was disconnected. The perfusion of the pulmonary trunk was performed by inserting a cannula in the pulmonary artery. It was initiated immediately after the first cardioplegia and the length of time was 30 minutes. The pulmonary perfusion pressure was strictly controlled by a digital manometer based on the mean pulmonary artery pressure measured before the median thoracotomy through the Swan-Ganz catheter. During the perfusion period of the pulmonary trunk, a probe ("Vent") was used for drainage of the left chambers, in order to avoid distension of them. The flow of systemic perfusion was based on the animal body surface and the perfusion flow from the pulmonary trunk was adjusted according to the level of pulmonary perfusion pressure defined previously. Soon after the CPB was removed, hemodynamic measurements were performed within the postoperative period.
In animals in the beating heart group II, after performing systemic therapeutic administration of heparin (4 mg / kg), the total CPB and the mechanical ventilation support were disconnected. The perfusion of the pulmonary trunk was started and maintained for 30 minutes. The perfusion pressure of the pulmonary trunk was strictly controlled by a digital manometer, and based on the mean pulmonary artery pressure, measured before the median thoracotomy through the Swan-Ganz catheter. During the period of pulmonary perfusion, a tourniquet was placed at both vena cavae and the right atrium was drained by a probe (Vent). Thus, the perfusion of the pulmonary trunk could only be made through the cannula inserted into the pulmonary trunk. The flow of systemic perfusion was based on the animal body surface, and the perfusion flow from the pulmonary trunk was adjusted according to the level of pulmonary perfusion pressure defined previously. Soon after the CPB removal, hemodynamic measurements were performed in the postoperative period.
In groups I and II, the pulmonary arterial perfusion was performed by an artificial line, which was derived from the recirculation line. Pulmonary perfusion with venous blood was made in turn from a derivation set up on the line between the venous reservoir and oxygenator. During the procedure, the mean arterial pulmonary pressure and the pulmonary perfusion flow were equivalent to 24.6 mmHg and 200 ml/min, respectively. There were no differences regarding the pressure of pulmonary perfusion using either venous or arterial blood. The systemic perfusion varied from 1.2 to 1.4 l/min/m2. The mean CPB time ranged from 35 to 40 minutes.
The CPB circuit used in the experiments, as well as the cardioplegia system and Unique Thymus oxygenator were manufactured by Nipro Brazil.
Hemodynamic assessment/BNP/Histologic analysis
Hemodynamic evaluations performed using the Swan-Ganz catheter was performed shortly after the CPB support removal. The following variables mean pulmonary artery pressure, and pulmonary vascular resistance was analyzed. Fragments of the infundibulum were collected to determine the expression of brain natriuretic peptide (BNP) and tissue histological pattern in the pre-operative periods (after thoracotomy and pericardiotomy, before installing CPB) and after 30 minutes of perfusion of the pulmonary trunk. The CPB withdrawal practically coincided with the end of 30 minutes of pulmonary perfusion. Tissue BNP tissue expression was analyzed by the method of real-time polymerase chain reaction (rt-PCR) and the histological examination of myocardial samples was done according to the hematoxylin and eosin technique.
The rt-PCR method was applied only when the tissue samples from each animal showed values of BNP expression minimally detectable. A specialized pathologist in myocardial inflammatory reaction performed the analysis independently and without prior knowledge about the various research subgroups. Furthermore, this pathologist has adopted semi-quantitative criteria to determine the intensity of different types of inflammatory lesions in the myocardium. The intensity of the lesions in each successive field was scored as 0 for no lesions, 1 for minimal, 2 for moderate, and 3 for severe. The extent of lesions was graded as 0 (absent), 1 (1-25% of tissue affected), 2 (26-50% of tissue affected), and 3 (<50% of tissue affected). The average score for successive fields represented the score for each individual sample [12].
Comparative analysis for hemodynamic study and the expression of myocardial BNP was made between pairs of subgroups within each main group, i.e., no comparisons were made between subgroups belonging to different major groups. Regarding the histological study, the pre- and postoperatively were compared individually for each subgroup, once the preoperative myocardial histological pattern could be confronted with the myocardial histological pattern after 30 minutes of pulmonary perfusion.
Statistical Analysis
The sample size for this experiment was estimated using the formula of Sturges and from this, the sample number 32 was considered minimally acceptable for this purpose. The statistical power of the small number of patients enrolled (n = 33) was 0.95 and for each one of the 16 elements in each major group, this power was 0.70. The subdivision of each main group into subgroups produced a low statistical power for the sample size in each subgroup, and it certainly should be considered an important limitation of this study. Normality was tested with the Kolmogorov-Smirnov test. When not normally distributed the Mann-Whitney U test was used to compare non-parametric data. The variables themselves with their values provided the data to prove the not normally distributed continuous data.
The Wilcoxon signed-rank sum test was used to establish a comparative analysis between two observational time frames (pre- and postoperative periods) for each subgroup individually (IA, IB, IC, IIA, IIB, IIC). The Mann-Whitney test was used to perform comparative analysis between two subgroups (IA x IB; IA x IC; IB x IC; IIA x IIB; IIB x IIC; IIB x IIC), focusing only the postoperative period. The Kruskal-Wallis test as used to concomitantly compare three subgroups among each other (IA x IB x IC; IIA x IIB x IIC), within each major group in the postoperative period. For the statistical tests, an alpha error of 5% was admitted. The
Statistical Package for Social Sciences (SPSS) version 13.0 software (SPSS, Chicago, IL, USA) were used to compute all the outcomes.
RESULTS
In the postoperative period, the values for the hemodynamic parameters determined through measurements obtained with the Swan-Ganz catheter showed significant changes with regard to mean pulmonary artery pressure and pulmonary vascular resistance among subgroups of animals whose lungs were perfused with controlled pressure and subgroups of animals whose lungs were not perfused with controlled perfusion pressure.
The comparative analysis between subgroups into the group I (cardioplegia) was characterized by postoperative values significantly lower from mean pulmonary arterial pressure and pulmonary vascular resistance in the subgroup of animals whose lungs were perfused with either arterial or venous blood, adopting the strategy of controlled rigorous perfusion pressure (Figure 1).
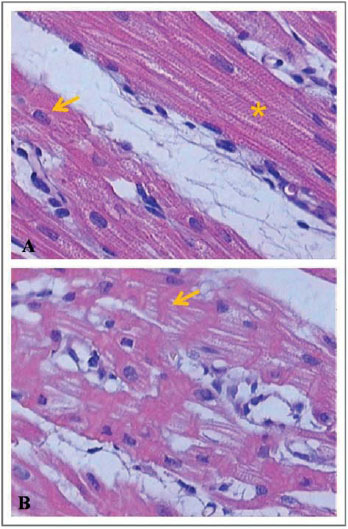
Fig. 1 - Postoperative comparison between subgroups of the cardioplegia group regarding the hemodynamic variables. IA - without pulmonary trunk perfusion; IB - perfusion with the pulmonary arterial blood; IC - pulmonary perfusion with venous blood; MPAP - mean pulmonary artery pressure in mmHg, PVR - pulmonary vascular resistance in dyne.sec.cm
5
In subgroups of the group II (beating heart) in which the lungs were perfused with arterial or venous controlled perfusion pressure, It has been detected a significantly lower postoperative value of the pulmonary vascular resistance (Figure 2).
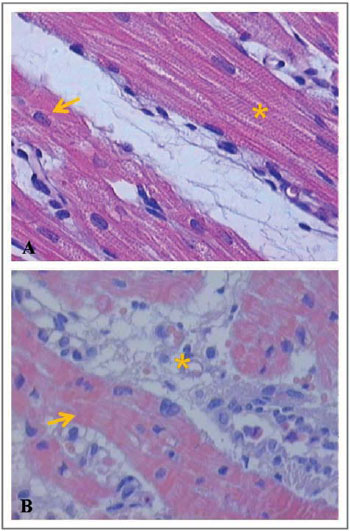
Fig. 2 - Postoperative comparison between subgroups of the beating heart group regarding the hemodynamic variables. IIA - without pulmonary trunk perfusion; IIB - perfusion with pulmonary arterial blood; IIC - pulmonary perfusion with venous blood; MPAP - mean pulmonary arterial pressure in mmHg, PVR - pulmonary vascular resistance in dyne.sec.cm
5
Regarding the expression of BNP in the right ventricular myocardium, it was considered only the tissue samples in which the quantitative BNP expression was minimally detectable by real-time PCR. Thus, samples of myocardial tissue that did not express minimally amounts of BNP detectable by the rt-PCR method were not included in this analysis. This generated the sample size variability presented for each subgroup studied. The BNP postoperative values were not significantly relevant for the subgroups of the group I, nor between subgroups of group II (Figure 3).
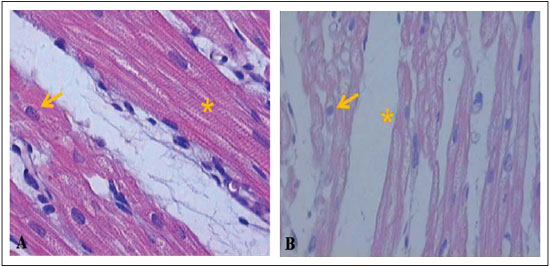
Fig. 3 - Expression of myocardial BNP in groups I and II. IA - without pulmonary trunk perfusion; IB - perfusion with pulmonary arterial blood; IC - pulmonary perfusion with venous blood; IIA - without pulmonary trunk perfusion; IIB - perfusion with pulmonary arterial blood; IIC - pulmonary artery perfusion with venous blood, BNP - brain natriuretic peptide in g / mL
Evaluations performed in the preoperative period and after 30 minutes of the pulmonary trunk perfusion in each subgroup individually, showed different myocardial lesions specially of inflammatory nature, such as interstitial edema, interstitial hemorrhage, cellular infiltration, contraction bands and areas of necrosis.
These lesions were measured using semi-quantitative criteria, as above-mentioned in Methods, and presented in a descriptive way for the subgroups of the cardioplegia group (Figure 4) and for subgroups of the beating heart group (Figure 5).
Next, Figures 6-8 illustrate and exemplify the main myocardial lesions, especially of inflammatory nature, which were found in different subgroups of the study population.
DISCUSSION
In heart surgery using CPB support, there is no sufficient and adequate perfusion of the pulmonary arteries, resulting in endothelial dysfunction. Because of pulmonary ischemia and reperfusion lesions, the surface receptors of inflammatory cells are stimulated, resulting in the activation of intracellular signalling cascades and in the subsequent positive regulation of transcription of several pro-inflammatory mediators, such as cytokines, chemokines and cell adhesion molecules [13-18].
In the study outcomes of Schlensak et al. [7], there was a significant decrease in bronchial artery flow in the initial phase of total CPB; this flow remained low until the end of CPB and returned to near normal levels during throughout the 60 minutes of reperfusion after CPB withdrawal. Histological analysis made by these authors revealed that the increase in alveolar septal thickness and the decrease in alveolar surface area were significantly attenuated by the controlled perfusion of pulmonary artery [7].
Gabriel et al. [19] demonstrated experimentally the advantages of the pulmonary trunk with controlled perfusion pressure using both arterial and venous blood. From the hemodynamic point of view, these authors observed a significant reduction in mean pulmonary artery pressure and pulmonary vascular resistance in the immediate postoperative period in animals that underwent perfusion controlled of the pulmonary trunk.
High levels of BNP are found in patients with ventricular dysfunction, acute myocardial infarction, unstable angina, hypertension, and ventricular hypertrophy. In patients with right ventricular pressure overload due to primary pulmonary hypertension and thromboembolism, BNP levels are higher than that in those patients with right ventricular volume overload due to atrial septal defect [20-22].
In patients with chronic thromboembolic pulmonary hypertension, BNP levels have been used as markers of primary pulmonary hypertension, and after thromboendarterectomy, levels of BNP and total pulmonary vascular resistance decreases. Patients who presenting left and right heart failure has shown values significantly higher of BNP compared to those who have presented only left heart failure.
In patients with chronic right ventricular pressure overload, the measurement of the left ventricular ejection fraction was negatively correlated with plasma BNP levels, suggesting that BNP levels are elevated in patients with right ventricular systolic dysfunction.
BNP is secreted primarily by the ventricles of the heart and has been used as a noninvasive marker of right ventricular dysfunction. Recent studies have shown that BNP levels increase in proportion to the degree of pulmonary hypertension and right ventricular dysfunction [23,24].
Nagaya et al. [11] showed that the preoperative plasma BNP levels are significantly high in patients with thromboembolic pulmonary hypertension. These authors emphasized that in the postoperative phase, plasma BNP levels correlated positively with mean pulmonary arterial pressure and negatively with cardiac output, thus demonstrating a strong positive correlation with pulmonary vascular resistance. The decrease in BNP levels correlated with a decrease in pulmonary vascular resistance after pulmonary thromboendarterectomy.
Although many studies suggest a correlation between BNP levels and variables, such as mean pulmonary artery pressure and pulmonary vascular resistance, there was no direct relationship in the present study between hemodynamic benefits associated with controlled perfusion of the pulmonary trunk and BNP expression in the right ventricular myocardium. The authors of this study hypothesize that the time of pulmonary perfusion, i.e., the duration of the controlled perfusion of the pulmonary trunk could constitute an important limiting factor for obtaining significant variations of the expression of myocardial BNP. Thus, the controlled pulmonary perfusion for a period of 30 minutes was not sufficient to promote substantial changes in the synthesis of BNP by the right ventricular myocytes.
Concerning the histological analysis of samples of the right ventricular myocardium, this study revealed that, in subgroups IA and IB, interstitial hemorrhage was significant in the postoperative period. In subgroups IB and IC, the presence of contraction bands and areas of myocardial necrosis was perceivable in the postoperative period.
Concerning the beating heart group, the subgroups IIA and IIC had significant myocardial lesions in the postoperative period, mainly expressed by areas of necrosis. In the subgroup B, there were no significant differences between the preoperative and postoperative periods, regarding the grading of inflammatory lesions. Based on these histological findings, it can be assumed that the inflammatory lesions found in the postoperative period of each group were due to pro-inflammatory factors related to cardiopulmonary bypass, regardless of whether the pulmonary trunk was perfused during the procedure.
Therefore, it becomes prominent again the question relative to a possible influence of time of controlled infusion of the pulmonary trunk, once the histological findings were correlated specifically with a continuous period of 30 minutes of perfusion of the pulmonary trunk.
In short, the controlled perfusion of the pulmonary trunk for 30 minutes, during heart surgery according to the experimental model produced, did not cause marked changes in expression of myocardial BNP in the immediate post-CPB period.
ACKNOWLEDGMENTS
The authors thank the Nipro Medical Ltda (Brasil) by indispensable grants and logistic support to carry out the present research. We also thank Euro Barros Couto Jr, the statistician due to his overcareful assessment of the research data, Prof. Dr. Márcia Marcelino de Souza Ishigai, by her dedication and engagement in interpreting the histological findings, Prof. Dr. Ismael Guerreiro Silva, by the molecular biology analyses, and Dr. Paulo Sérgio Venerando Silva, the veterinary physician by his valuable support on anestesia, hemodynamic monitorization and in performing the exams in the study animals.
REFERENCES
1. Buckberg GD. Myocardial temperature management during aortic clamping for cardiac surgery. Protection, preoccupation, and perspective. J Thorac Cardiovasc Surg. 1991;102(6):895-903. [MedLine]
2. Kassab GS, Kostelec M, Buckberg GD, Covell J, Sadeghi A, Hoffman JI. Myocardial protection in the failing heart: II. Effect of pulsatile cardioplegic perfusion under simulated left ventricular restoration. J Thorac Cardiovasc Surg. 2006;132(4):884-90. [MedLine]
3. Dearani JA, Axford TC, Patel MA, Healey NA, Lavin PT, Khuri SF. Role of myocardial temperature measurement in monitoring the adequacy of myocardial protection during cardiac surgery. Ann Thorac Surg. 2001;72(6):S2235-43.
4. Nicolini F, Beghi C, Muscari C, Agostinelli A, Maria Budillon A, Spaggiari I, et al. Myocardial protection in adult cardiac surgery: current options and future challenges. Eur J Cardiothorac Surg. 2003;24(6):986-93. [MedLine]
5. Cressoni ES, Avanci LE, Braile DM, Cicogna AC, Oliveira APML, Gerez MAE, et al. Proteção miocárdica ao coração hipertrofiado: o eterno desafio. Rev Bras Cir Cardiovasc. 2008;23(1):97-107. [MedLine] View article
6. Mota AL, Rodrigues AJ, Évora PRB. Circulação extracorpórea em adultos no século XXI. Ciência, arte ou empirismo? Rev Bras Cir Cardiovasc. 2008;23(1):78-92. [MedLine] View article
7. Schlensak C, Doenst T, Preusser S, Wunderlich M, Kleinschmidt M, Beyersdorf F. Bronchial artery perfusion during cardiopulmonary bypass does not prevent ischemia of the lung in piglets: assessment of bronchial artery blood flow with fluorescent microspheres. Eur J Cardiothorac Surg. 2001;19(3):326-31.
8. Siepe M, Goebel U, Mecklenburg A, Doenst T, Benk C, Stein P, et al. Pulsatile pulmonary perfusion during cardiopulmonary bypass reduces the pulmonary inflammatory response. Ann Thorac Surg. 2008;86(1):115-22. [MedLine]
9. Inokawa H, Sevala M, Funkhouser WK, Egan TM. Ex-vivo perfusion and ventilation of rat lungs from non-heart-beating donors before transplant. Ann Thorac Surg. 2006;82(4):1219-25. [MedLine]
10. Sievers HH, Freund-Kaas C, Eleftheriadis S, Fischer T, Kuppe H, Kraatz EG, et al. Lung protection during total cardiopulmonary bypass by isolated lung perfusion: preliminary results of a novel perfusion strategy. Ann Thorac Surg. 2002;74(4):1167-72.
11. Nagaya N, Nishikimi T, Okano Y, Uematsu M, Satoh T, Kyotani S, et al. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol. 1998;31(1):202-8. [MedLine]
12. Delmonte C, Capelozzi VL. Morphologic determinants of asphyxia in lungs: a semiquantitative study in forensic autopsies. Am J Forensic Med Pathol. 2001;22(2):139-49. [MedLine]
13. Boyle EM Jr, Pohlman TH, Johnson MC, Verrier ED. Endothelial cell injury in cardiovascular surgery: the systemic inflammatory response. Ann Thorac Surg. 1997;63(1):277-84. [MedLine]
14. Fan X, Liu Y, Wang Q, Yu C, Wei B, Ruan Y. Lung perfusion with clarithromycin ameliorates lung function after cardiopulmonary bypass. Ann Thorac Surg. 2006;81(3):896-901. [MedLine]
15. Real JM, Marques MM, Camargo AA, Deheinzelin D, Dias AA. The role of the acute phase protein PTX3 in the ventilator-induced lung injury. Eur Respir J. 2007;30(Suppl 51):456s. [MedLine]
16. Liu Y, Wang Q, Zhu X, Liu D, Pan S, Ruan Y, et al. Pulmonary artery perfusion with protective solution reduces lung injury after cardiopulmonary bypass. Ann Thorac Surg. 2000;69(5):1402-7. [MedLine]
17. Shimamoto A, Pohlman TH, Shomura S, Tarukawa T, Takao M, Shimpo H. Toll-like receptor 4 mediates lung ischemia-reperfusion injury. Ann Thorac Surg. 2006;82(6):2017-23. [MedLine]
18. Li C, Ha T, Kelley J, Gao X, Qiu Y, Kao RL, et al. Modulating Toll-like receptor mediated signaling by (1->3)-beta-D-glucan rapidly induces cardioprotection. Cardiovasc Res. 2004;61(3):538-47. [MedLine]
19. Gabriel EA, Fagionato Locali R, Katsumi Matsuoka P, Santiago Almeida L, Guerreiro Silva I, Capelozzi VL, et al. Lung perfusion during cardiac surgery with cardiopulmonary bypass: is it necessary? Interact Cardiovasc Thorac Surg. 2008;7(6):1089-95. [MedLine]
20. Yap LB, Mukerjee D, Timms PM, Ashrafian H, Coghlan JG. Natriuretic peptides, respiratory disease, and the right heart. Chest. 2004;126(4):1330-6. [MedLine]
21. Krishnaswamy P, Lubien E, Clopton P, Koon J, Kazanegra R, Wanner E, et al. Utility of B-natriuretic peptide levels in identifying patients with left ventricular systolic or diastolic dysfunction. Am J Med. 2001;111(4):274-9. [MedLine]
22. Omland T,Aakvaag A, Bonarjee VV, Caidahl K, Lie RT, Nilsen DW, et al. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation. 1996;93(11):1963-9. [MedLine]
23. Nakanishi K, Tajima F, Itoh H, Nakata Y, Osada H, Hama N, et al. Changes in atrial natriuretic peptide and brain natriuretic peptide associated with hypobaric hypoxia-induced pulmonary hypertension in rats. Virchows Arch. 2001;439(6):808-17. [MedLine]
24. Zhao L, Mason NA, Strange JW, Walker H, Wilkins MR. Beneficial effects of phosphodiesterase 5 inhibition in pulmonary hypertension are influenced by natriuretic Peptide activity. Circulation. 2003;107(2):234-7. [MedLine]
Article receive on Monday, April 26, 2010



 All scientific articles published at bjcvs.org are licensed under a Creative Commons license
All scientific articles published at bjcvs.org are licensed under a Creative Commons license