ABSTRACT
Objective: Safety, feasibility and early myocardial angiogenic effects evaluation of transthoracic intramyocardial phVEGF165 administration for refractory angina in no option patients. Methods: Cohort study, in which 13 patients with refractory angina under optimized clinical treatment where included, after cineangiograms had been evaluated and found unfeasible by surgeon and interventional cardiologist. Intramyocardial injections of 5mL solution containing plasmidial VEGF165 where done over the ischemic area of myocardium identified by previous SPECT/Sestamibi scan. Evaluations included a SPECT scan, stress test, Minnesotta QOL questionnaire and NYHA functional class and CCS angina class determinations. Results: There were no deaths or new interventions during the study period. There were no significant variations in SPECT scans, QOL scores and stress tests results during medical treatment in the included patients. After the 3rd post operative month, there was improvement in SPECT segmental scores, SSS (18.38±7.51 vs. 15.31±7.29, P=0.003) and SRS (11.92±7.49 vs. 8.53±6.68, P=0.002). The ischemic area extension, however, had non-significant variation (23.38±13.12% vs. 20.08±13.88%, P=0.1). Stress tests METs varied from 7.66±4.47 pre to 10.29±4.36 METs post-op (P=0.08). QOL score improved from 48.23±18.35 pre to 30.15±20.13 post-op points (P=0.02). NYHA class was 3.15±0.38 pre vs. 1.77±0.83 post-op (P=0.001) and angina CCS class, 3.08±0.64 vs. 1.77±0.83 (P=0.001). Conclusions: Intramyocardial VEGF165 therapy for refractory angina, in this small trial of no option patients, resulted feasible and safe. Early clinical and scintilographic data showed improvements in symptoms and myocardial perfusion, with regression of ischemia severity in treated areas.
RESUMO
Objetivo: Avaliar a segurança, viabilidade e efeitos iniciais, clínicos e sobre a perfusão miocárdica, da administração intramiocárdica, transtorácica, de VEGF 165 plasmidial em pacientes com doença arterial coronariana avançada e angina refratária, não passíveis de revascularização percutânea e cirúrgica. Métodos: Ensaio clínico fase I/II. Treze pacientes cardiopatas isquêmicos com angina refratária apesar de tratamento medicamentoso máximo por no mínimo seis meses, não passíveis de revascularização cirúrgica ou por cateter foram submetidos a injeções intramiocárdicas de 2000µg VEGF 165 plasmidial. Os pacientes foram avaliados por cintilografia miocárdica, teste ergométrico, questionário de qualidade de vida (Minnesota) e determinação das classes de insuficiência cardíaca (NYHA) e angina (CCS). Resultados: Não houve óbitos ou reintervenções. Durante o período de tratamento medicamentoso máximo, não se observou diferenças em cintilografias miocárdicas, testes ergométricos e questionários de qualidade de vida, ainda, houve tendência a piora das classes NYHA (P=0,05) e CCS (P=0,05). Três meses após intervenção, observou-se melhora dos escores cintilográficos SSS (18,38±7,51 vs. 15,31±7,29, P=0,003) e SRS (11,92±7,49 vs. 8,53±6,68, P=0,002), porém não na proporção da extensão da área de miocárdio isquêmico (23,38±13,12% vs. 20,08±13,88%, P=0,1). Houve tendência a melhora dos METs nas ergometrias (7,66±4,47 vs. 10,29±4,36, P=0,08), melhora do escore de qualidade de vida (48,23±18,35 vs. 30,15±20,13; P=0,02) e das classes NYHA (3,15±0,38 vs. 1,77±0,83, P=0,001) e CCS (3,08±0,64 vs. 1,77±0,83, P=0,001), no mesmo período. Conclusões: A terapia demonstrou-se segura e viável nesta série de pacientes. Os resultados iniciais tendem a demonstrar melhora na gravidade da angina e redução da intensidade da isquemia miocárdica.
INTRODUCTION
The demonstration that some vascular growth factors have the potential to induce angiogenesis in ischemic tissue [1-3] has stimulated research into new treatment techniques for patients with coronary artery disease (CAD). This is due to the possibility of inducing myocardial angiogenesis and establishing collateral circulation, especially in cases of refractory angina to conventional therapy and unfeasible techniques for traditional CABG and PCI.
The complex cascade of events that occurs during neovascularization in response to ischemia involves several growth factors and receptors [4]. The vascular endothelial growth factor (
vascular endothelial growth factor - VEGF) has been the focus of several studies [5,6] and its effects are being tested in patients with severe CAD [7-20]. Studies use different doses, vectors and ways of administration of VEGF. Clinical trials bring controversial results, many showing evidence of clinical improvement and angiogenesis [8-19] and others showing no differences in myocardial perfusion when compared to their controls [7,20]. Thus, although promising, remain still not completely clear the clinical effects on the myocardium vascularization of the therapy with VEGF in its various forms and ways of administration, justifying further studies.
This clinical assay aims to evaluate the safety, feasibility and initial clinical effects (featuring a clinical assay phase I / II), under myocardial perfusion, intramyocardial, transthoracic administration of plasmid VEGF 165 in patients with advanced CAD and refractory angina, which are not qualified for surgery and percutaneous revascularization. We emphasize that we used for the first time, a plasmid entirely produced in Brazil.
METHODS
Design
Cohort study Phase I, which was registered at
Clinical Trial.gov under number NCT00744315. In this, severe ischemic heart disease patients, not qualifying for revascularization surgery or catheter after a review of coronary angiography and cardiovascular surgeon independently, were selected to optimize the period of clinical treatment for 6 months or more before receiving intramyocardial injections of VEGF 165 via left minithoracotomy. All patients had refractory angina although they were subjected to maximum medical treatment in specific clinics.
Sample
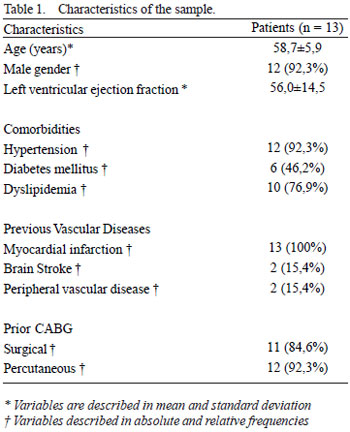
Thirteen patients were selected with refractory angina (Table 1) originated from 134 cases evaluated from the following eligibility criteria: patients with refractory angina to maximum optimal medical treatment and whose coronary angiography was examined by a cardiovascular surgeon independently judged as untreatable by percutaneous or surgical techniques, the aged under 75 years, left ventricular ejection fraction exceeding 25%, symptoms of angina and/or heart failure despite maximum medical treatment, absence of diagnosed neoplastia and maintenance of myocardial hypoperfusion in control scintigraphy after the period of optimal pharmacological treatment.
Plasmid vector
The plasmid vector used was developed in Brazil by one of the authors (S.W.H.), Federal University of São Paulo and it was produced commercially by a technology company in Brazil, Excellion, Petropolis, RJ. The structure of the PEXHV5 consists of intron 1 and its promoter with a signal of cytomegalovirus (CMV). Human DNA of VEGF165 was inserted between the CMV promoter and bovine sequence poliA. This vector also contains pUC origin and resistance sequence to kanamycin for bacterial spread.
Intervention
The patients underwent surgical intervention in centers specialized in cardiovascular surgery under general anesthesia. Heart was exposed by an incision of approximately 5 cm in the 4th or 5th left intercostal space, according to the heart area to be treated, performing extensive pericardiotomy and repair of the pericardium (Figure 1). The area to receive injections of the plasmid vector solution was guided by a study of preoperative myocardial scintigraphy. Ten points of the ischemic myocardium received, under direct vision, a total of 2,000 mg of VEGF 165, diluted in a total of five milliliters of saline. For the injection it was used a scalp 25F. Before the thoracotomy, a pleural drain was placed and maintained for 12h. The postoperative pain was treated with intravenous analgesics and intercostal block.
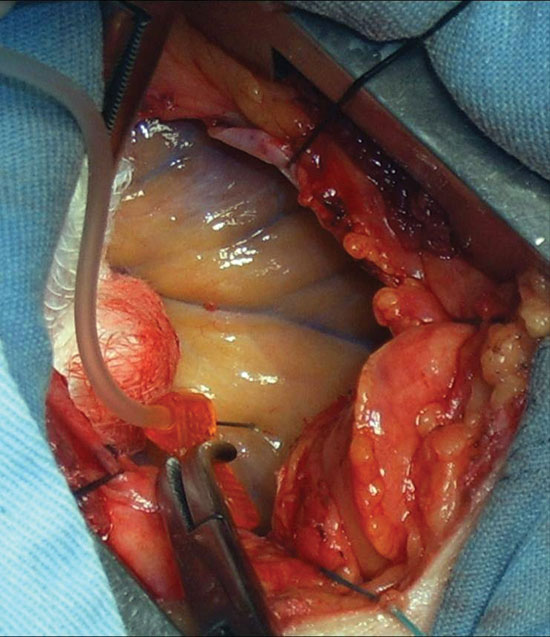
Fig.1 - Pericardial window performed for intramyocardial injection of VEGF 165
Evaluations were conducted for selection of patients in a cardiology clinic. Those included were submitted to evaluations at the time of inclusion, after at least 6 months of maximum drug treatment in the immediate preoperative period, after completing 1 and 3 months postoperatively. These assessments included:
Tomographic scintigraphy
Myocardial scintigraphy with tomographic incisions ("
Single Photon Emission Computed Tomography"- SPECT), synchronized with the ECG was performed with Tc-99m sestamibi technique and two day technique (rest/stress). All patients underwent pharmacological stress with dipyridamole in a dose of 0.56 mg / kg in 4 minutes. The injection of the radiotracer was carried out in the seventh minute of the start of the infusion of dipyridamole. The images were acquired on a gamma camera of two detectors, using a high-resolution low-energy collimator, 180 º noncircular orbit starting in right anterior oblique projection at 45 ° and ending at the oblique left posterior projection being obtained 64 projections with a matrix of 64 X 64. Images were reconstructed using conventional filtered backprojection after noise reduction with a low-pass filter. The stress and rest studies were reconstructed using a Butterworth filter with critical frequency of 0.5 Nyquist and an order of 5.
The
Emory Cardiac Toolbox software was used to locate the boundaries of the left ventricle (verified later by an operator) and to obtain the values of ejection fraction and ventricular volumes. The extent and severity of the ischemic area were calculated with the use of Cedars-Sinai QPS software. In that software, the left ventricle is divided into segments and each one receives a score from 0 to 4 (0 = normal perfusion and 4 = absence of perfusion). Severity scores are obtained: summed stress score (SSS), summed rest score (SRS) and difference score (SDS). The extent of the ischemic area is calculated by comparing the perfusion of the patient with a database of normal patients. The examination final result was obtained by consensus between two expert examiners.
Exercise Stress Test
A Naughton protocol was used, which provides about o1 MET per stage. This protocol is designed for individuals with significant physical limitations, especially the elderly and sedentary, as well as recent developments in acute myocardial infarction and in patients with stable heart failure. The protocol begins with belt speed of 1.0 mph with no inclination, with the purpose of heating. Afterwards, is set to 2.0 mph, keeping zero elevation. From this stage, the speed is fixed at 2 mph and the elevation of the ramp suffers 3.5% increments every 3 minutes.
Quality of Life score by applying the Minnesota questionnaire (QOL) - Brazilian version
The questionnaire of quality of life by Minnesota is comprised of 21 questions relating to limitations which are often associated with how much heart failure (HF) prevents patients from living as they would like. Considering the last month to answer the questions. The scale of responses to each question ranges from 0 (no) to 5 (too much), where 0 represents no limitation and 5, maximum limitation. This questionnaire was developed specifically for HF, making it closer to the reality of this type of patient.
Heart failure class (NYHA)
As follows:
Class I - No limitation during ordinary activity; Class II - Some limitation due to dyspnea or fatigue during moderate stress or moderate exercise; Class III - Symptoms with minimal exertion that interfere with daily activities; Class IV - Inability to perform any physical activity, dyspnea at rest.
Angina class (CCS)
As follows:
Class I - Routine physical activity such as walking or climbing stairs does not cause angina. Angina occurs with prolonged and intense physical exertion; Class II - Slight limitation for daily activities: angina occurs to the patient when walking or climbing stairs rapidly, walking uphill, walking or climbing stairs after meals, or in cold or windy climates, under emotional stress, or just a few hours after awakening. Angina occurs after walking for two plane blocks or climbing more flight of stairs under normal conditions; Class III - Limitation in usual activities: Angina occurs when walking a plane block or climbing a flight of stairs; Class IV - Inability to perform any normal activity without discomfort, anginal symptoms may be present at rest.
Ethical considerations
The study was designed according to the Guidelines and Norms Regulating Research Involving Human Subjects (Declaration of Helsinki World Medical Association, 1964) and it was approved by the local Biosafety and Research Ethics Committees (protocol number 3849/06), the National Ethics Committee (CONEP) and the National Technical Commission on Biosafety (CTNBio) as well as the supply laboratory of plasmid vector, and the surgical block where procedures were performed. All patients agreed to participate in the study voluntarily and signed a consent form.
Statistical analysis
Analyses were performed using the statistical program PAWS version 18.0. Continuous data are expressed as mean ± standard deviation and categorical variables in absolute and relative frequencies. The groups were compared using chi-square (Pearson chi-square) for categorical variables and Student's t test for continuous variables. When there were three means obtained from samples in a row from continuous variables with normal distribution, we applied the analysis of variance and Bonferroni correction, and for categorical data we used the nonparametric Friedman test, followed by its multiple comparison test. It was considered statistically significant a
P <0.05.
RESULTS
Safety and feasibility
The main variables analyzed in the trans-and postoperatively, as times of hospitalization, surgery and thoracic drainage are presented in Table 2. There were no cases of death or surgical intervention in this period. There was one case of prolonged postoperative hospital stay due to decompensation of
diabetes mellitus. In addition, one patient had ventricular premature beats in the immediate postoperative period. It is noteworthy that the same patient had presented the same arrhythmia in a prior exercise test, and there was spontaneous reversion.
Another patient developed bacteremia associated with pneumonia within 12 hours postoperatively. However, the cultural analysis of the plasmid used in this patient showed no bacterial or fungal growth until the fifth day of observation in a suitable medium. This same patient required assistance in an emergency room in the 4th month after the intervention due to stress crisis caused by family illness, without being detected change in the cardiologic clinical situation. The patient was managed medically without the need for hospitalization. Another patient required hospitalization for signs of heart failure, which was also handled clinically.
Myocardial scintigraphy
The preliminary analysis showed scintigraphic reduction in the number of ischemic segments, under stress and rest. Although the total proportion of ischemic area of the ventricular myocardium after treatment have shown no significant trend of reduction, i.e., it was 23.38 + -13.12% of the left ventricle in the immediate pre-operatively and after 3 months it was 20. 08 ± 13.88% (
P = 0.1) when evaluated the scores of segmental myocardial ischemia, SSS (18.38 ± 7.51 to 15.31 ± 7.29,
P = 0.003) and SRS (for 11.92 ± 7.49 to 8.53 ± 6.68,
P = 0.002), we observed a significant reduction in the number of ischemic segments at three months after gene therapy with VEGF 165. The greatest benefit was seen after the first month of treatment. The difference scores did not change significantly (Figure 2).
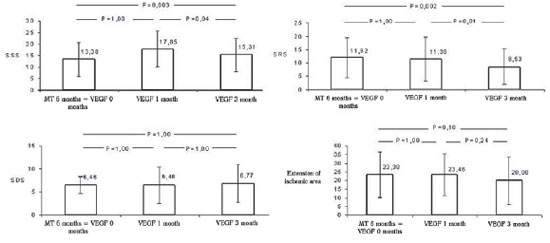
Fig.2 - Scintigraphy - mean of extensions of the ischemic area and scores of segmental ischemia on stress, rest and differential presented by the patients during the study period. MT 6 months = VEGF 0 months: pharmacotherapy in the sixth month = immediately before the intervention with VEGF 165, VEGF 1 month, one month after the intervention of VEGF 165, VEGF 3meses: three months after the intervention of VEGF 165. SSS = summed stress score, SRS = summed rest score, SDS = summed difference score. Values expressed as mean and standard deviation. Statistical test used: ANOVA for paired samples, followed by post hoc Bonferroni
Figure 3 illustrates the variation of myocardial perfusion evaluated by scintigraphy of one of the patients included in the protocol. There is an improvement of perfusion of the lower myocardial wall three months after treatment.
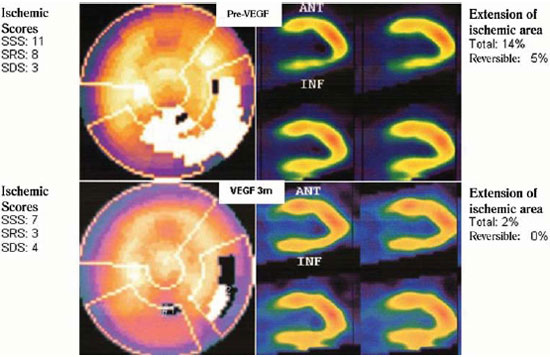
Fig.3 - Picture of myocardial scintigraphy of one case study. Case: 54 year old male, hypertensive, diabetic, with a history of percutaneous coronary and myocardial revascularization. Received 2000ìg of plasmid VEGF 165 in myocardial wall below
In the evaluation performed during the clinical treatment there were no differences in METs obtained in exercise tests, which were 7.02 ± 4.54 versus 7.66 METs inclusion ± 4.47 METs at the immediate preoperative (
P = 0.36). However, it was observed a tendency, not statistically significant, to the increase in METs achieved by the patients three months after the application of gene therapy, i.e., from 7.66 ± 4.47 METs to 10.29 ± 4.36 METs (
P = 0.08) (Figure 4).
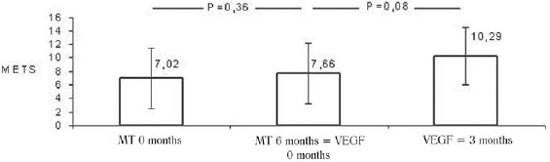
Fig.4 - Stress test - Average METs obtained by patients during the study period. MT 0 months: drug therapy at baseline; MT 6 months = VEGF 0 months: pharmacotherapy in the sixth month = immediately before the intervention with VEGF 165, VEGF 3meses: three months after the intervention of VEGF 165. Values expressed as mean and standard deviation. Statistical test used: ANOVA for paired samples, followed by post hoc Bonferroni
During the period of maximum medical treatment, there was no difference in scores for quality of life of patients (49.77 ± 20.84 in the inclusion vs. 48.23 ± 18.35 in the immediate preoperative,
P = 1.00). After 3 months of gene therapy it was demonstrated a significant decrease in the score of points obtained in the questionnaire of quality of life, i.e., improvement of this variable, from 48.23 ± 18.35 to 30.15 ± 20.13 points (
P = 0.02) (Figure 5).
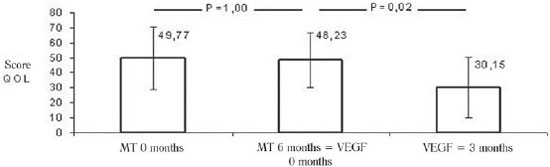
Fig.5 - Average scores of patients in a questionnaire about quality of life (QOL Minn.) during the study period. TM 0 months: drug therapy at baseline, MT 6 months = VEGF 0 months: pharmacotherapy in the sixth month = immediately before the intervention with VEGF 165, VEGF 3meses: three months after the intervention of VEGF 165. Values expressed as mean and standard deviation. Statistical test used: ANOVA for paired samples, followed by post hoc Bonferroni
It was demonstrated significant improvement in the median grade of heart failure (NYHA) of the patients three months after gene therapy (median 3 and interquartile interval 3-3 preoperatively to median 2 and interquartile interval 1 to 2.5 at 3 months,
P = 0.001), while the same was not observed during maximum drug treatment than in the previous period, at which the medians and interquartile intervals varied from 3 (2-3) at the start of drug treatment to 3 (3-3) at the end of it. Likewise, there was no difference in angina class of patients during clinical treatment (median 3 and interquartile interval 2-3 at the baseline to the median and interquartile interval 3-3.5 at the end of treatment). However, when the variable was examined three months after gene therapy, there was a reduction in the median angina class (median 3 and interquartile interval 3-3.5 preoperatively to median 2 and interquartile interval 1-2.5 at 3 months,
P = 0.001) (Figure 6).
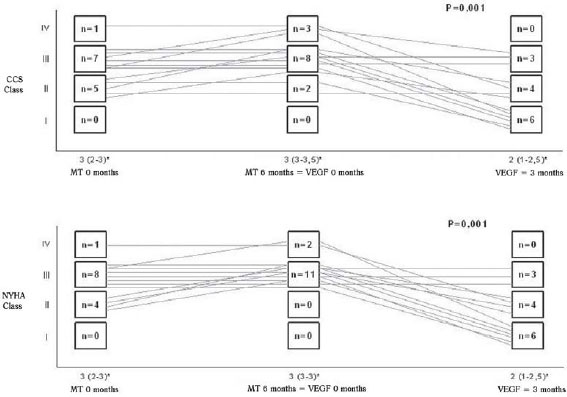
Fig.6 - Variation of the classes of heart failure (NYHA) and angina (CCS) patients during the study period. * Variable described as median and interquartile range. TM 0 months: drug therapy at baseline, MT 6 months = VEGF 0 months: pharmacotherapy in the sixth month = immediately before the intervention with VEGF 165, VEGF 3 months: three months after the intervention of VEGF 165. Statistical test used: Friedman test followed by his multiple comparisons
In the individual clinical assessment, we found that nine patients had improvement in symptoms, while four reported no improvement. There was no case of worsening after clinical gene therapy. Also, during clinical treatment, five patients reported worsening of symptoms and eight reported no difference from it.
DISCUSSION
The application of intramyocardial plasmid VEGF 165 in patients with refractory angina for ischemic cardiac disease with no other alternative therapy has shown to be feasible and safe in this series. Other authors have reported the safety procedures [19-21]. However, this information is important because it was done in our midst and with technical peculiarities of the surgical procedure, and, previously, only assays were performed in Brazil [23,24]. Furthermore, it was first used in a clinical assay a plasmid vector produced entirely in Brazil. It is known of the extreme difficulties in obtaining such vectors for clinical research by firms and international laboratories.
Minor complications occurred in only one patient. Although there were two hospital admissions in the followup period, were necessary only noninvasive procedures for resolution of the clinical states, as appropriate medical treatment.
A previous study [17] demonstrated the safety and bioactivity of gene transfer of VEGF in ischemic myocardium by human plasmid vector (phVEGF165) by minithoracotomy in a series of 20 patients with refractory angina compared with optimized drug therapy. Another study [15], in order to evaluate the safety and efficacy of VEGF165 via intramyocardium reported as an effective method for treatment in patients with chronic and advanced CAD even as a complementary treatment to medication. Furthermore, a randomized phase II [7], double-blind, placebo-controlled, multicenter, tested phVEGF-A165 plasmid via direct percutaneous intramyocardial in 80 patients with severe CAD, demonstrating safety and tolerability of the intervention.
We emphasize that our study did not use a parallel control group. We chose the model of temporal control, where the patient is his own control in the period prior to treatment due to the heterogeneity and variability of coronary artery disease, which makes it impossible to adequately compare the groups, mainly in small series. Nevertheless, all patients in the study continued for a minimum period of six months under maximum drug therapy before the intervention, which would be the option for patients who were not subjected to it. Hence, it was possible to compare the outcomes studied during the maximum drug therapy and after therapy with VEGF 165, with each patient, as stated above, control of himself. In any event, certainly it is not ruled out the possible influence of placebo effect in these results.
When evaluated the scintigraphic images, we observed improvement in perfusion scores in the treated area for three months after injection of plasmid VEGF 165, whereas in the scintigrams obtained after one month, this improvement was not evident. While segmental scores of ischemia on stress and at rest, respectively SSS and SRS, which assess both the extent and intensity of myocardial ischemia, showed improvement of myocardial perfusion, the total extent of infarction with scintigraphic signs of ischemia on the individual observation did not change during the period.
The proposed treatment aims to improve perfusion in regions with viable myocardium and not recovering inactive areas. Thus, we attribute the improvement in scintigraphic scores to the decrease in severity of ischemia in the treated areas. It can be understood that the improvement evident in the rest scintigraphy in this series of patients represents better myocardial perfusion, but it is still insufficient to complete reperfusion, for it remains the evidence of ischemia on stress, although it may be considered less than before. Either way, it represents a clear angiogenic effect of gene therapy used on these areas of myocardium.
Our study used VEGF 165 plasmid as sole therapy. Other previous reports used the same therapy, but in association with coronary artery bypass surgery, among others [7-16,19- 21]. Two previous studies [17,18] similar to ours in that respect showed controversial results, one showing an improvement of myocardial perfusion and angina [17] and another only improvement in angina [18]. However, they were also small series of patients and used different doses of VEGF 165.
Recent randomized clinical assay testing gene therapy combined with CABG surgery plus oral supplementation of L-arginine has shown promising results in the association, implying that endothelial dysfunction might be associated with the pathophysiology of this class of patients. Therefore, supplementation of L-arginine could induce increased bioavailability of nitric oxide being in favor of angiogenesis [19]. However, there is evidence that oral supplementation of L-arginine may not be safe in the treatment of acute myocardial infarction with ST-segment elevation [22]. Another recent study [20], randomized, multicenter, showed no significant difference in the scores of myocardial ischemia between the treated and control groups. Nonetheless, unlike the previous and our own study, this one used the electromechanical mapping technique by the system NOGA for injection of the vector.
The assessment of quality of life in this series showed improvement three months after the application of gene therapy with VEGF 165, and there had been no difference of the same variable as the patients remained under maximum drug therapy. A previous study [15] showed similar results in a series of 22 patients who received VEGF 165. Likewise, the functional classes of heart failure (NYHA) and angina (CCS) presented by the patients three months after therapy with VEGF 165 were significantly reduced. During the period of maximum drug therapy, we could observe even worsening of symptoms in some cases, which can be interpreted as the natural evolution of ischemic disease in terminal phase, without alternative therapy. Previous randomized clinical assay [10] had already shown similar results. In this assay, early in the follow-up all patients were classified in class III or IV of angina, and after 12 weeks it showed that the same variable was significantly reduced in patients who received phVEGF2. In contrast, the class of angina was unchanged in the control group.
It is considered that the possible interference of the placebo effect in this series of patients can change their perception about the symptoms of the disease, making it a confounder of the results. On the other hand, the scintigraphic improvements demonstrated in myocardial ischemic areas and the trend towards improvement in METs obtained by patients in the exercise tests may be indicative of relevance of the results identified in the scores of quality of life and classes NYHA and CCS. One must bear in mind also that when these scores were applied before and after maximum drug treatment, to which patients were submitted, no significant differences were observed, agreeing with the same hypothesis.
CONCLUSIONS
Gene therapy with VEGF 165 plasmid has proven to be safe and feasible in this patient sample. The initial clinical results appear to show improvement in the intensity of ischemia in areas of treated myocardium three months after treatment. This series of patients showed improvements in quality of life, and the classes of angina and heart failure in the same period. Although this study has been conducted in a small series of patients, partial results appear promising, stimulating research on this new treatment modality.
REFERENCES
1. Kastrup J. Therapeutic angiogenesis in ischemic heart disease: gene or recombinant vascular growth factor protein therapy? Curr Gene Ther. 2003;3(3):197-206. [MedLine]
2. Isner JM. Myocardial gene therapy. Nature. 2002;415(6868):234-9. [MedLine]
3. Lewis BS, Flugelman MY, Weisz A, Keren-Tal I, Schaper W. Angiogenesis by gene therapy: a new horizon for myocardial revascularization? Cardiovasc Res. 1997;35(3):490-7. [MedLine]
4. Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49(3):507-21. [MedLine]
5. Klagsbrun M. The fibroblast growth factor family: structural and biological properties. Prog Growth Factor Res. 1989;1(4):207-35. [MedLine]
6. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9-22. [MedLine]
7. Kastrup J, Jørgensen E, Ruck A, Tägil K, Glogar D, Ruzyllo W, et al; Euroinject One Group. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris. A randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol. 2005;45(7):982-8. [MedLine]
8. Eppler SM, Combs DL, Henry TD, Lopez JJ, Ellis SG, Yi JH, et al. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin Pharmacol Ther. 2002;72(1):20-32. [MedLine]
9. Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, et al. Pharmacological treatment of coronary artery disease with recombinant ? broblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002;105(7):788-93. [MedLine]
10. Losordo DW, Vale PR, Hendel RC, Milliken CE, Fortuin FD, Cummings N, et al. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002;105(17):2012-8. [MedLine]
11. Sylvén C, Sarkar N, Ruck A, Drvota V, Hassan SY, Lind B, et al. Myocardial Doppler tissue velocity improves following myocardial gene therapy with VEGF-A165 plasmid in patients with inoperable angina pectoris. Coron Artery Dis. 2001;12(3):239-43. [MedLine]
12. Rosengart TK, Lee LY, Patel SR, Sanborn TA, Parikh M, Bergman GW, et al. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically signi?cant severe coronary artery disease. Circulation. 1999;100(5):468-74. [MedLine]
13. Stewart DJ, Hilton JD, Arnold JM, Gregoire J, Rivard A, Archer SL, et al. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF(121) (AdVEGF121) versus maximum medical treatment. Gene Ther. 2006;13(21):1503-11. [MedLine]
14. Bokeriya LA, Golukhova EZ, Eremeeva MV, Aslanidi IP, Merzlyakov VY, Georgiev GP, et al. Use of human VEGF(165) gene for therapeutic angiogenesis in coronary patients: first results. Cell Tech Biol Med. 2005;3:123-30.
15. Kolsut P, Malecki M, Zelazny P, Teresinska A, Firek B, Janik P, et al. Gene therapy of coronary artery disease with phvegf165: early outcome. Kardiol Pol. 2003;59(11):373-84. [MedLine]
16. Vale PR, Losordo DW, Milliken CE, Maysky M, Esakof DD, Symes JF, et al. Left ventricular electromechanical mapping to assess efficacy of phVEGF(165) gene transfer for therapeutic angiogenesis in chronic myocardial ischemia. Circulation. 2000;102(9):965-74. [MedLine]
17. Symes JF, Losordo DW, Vale PR, Lathi KG, Esakof DD, Mayskiy M, et al. Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease. Ann Thorac Surg. 1999;68(3):830-6.
18. Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation.1998;98(25):2800-4. [MedLine]
19. Ruel M, Beanlands RS, Lortie M, Chan V, Camack N, deKemp RA, et al. Concomitant treatment with oral L-arginine improves the efficacy of surgical angiogenesis in patients with severe diffuse coronary artery disease: the Endothelial Modulation in Angiogenic Therapy randomized controlled trial. J Thorac Cardiovasc Surg. 2008;135(4):762-70.
20. Stewart DJ, Kutryk MJ, Fitchett D, Freeman M, Camack N, Su Y, et al; NORTHERN Trial Investigators. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol Ther. 2009;17(6):1109-15. [MedLine]
21. Ripa RS, Wang Y, Jørgensen E, Johnsen HE, Hesse B, Kastrup J. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. Eur Heart J. 2006;27(15):1785-92. [MedLine]
22. Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, et al. L-arginine therapy in acute myocardial infarction: the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295(1):58-64. [MedLine]
23. Kalil RA, Teixeira LA, Mastalir ET, Moreno P, Fricke CH, Nardi NB. Experimental model of gene transfection in healthy canine myocardium: perspectives of gene therapy for ischemic heart disease. Arq Bras Cardiol. 2002;79(3):223-32. [MedLine]
24. Furlani AP, Kalil RA, Castro I, Cañedo-Delgado A, Barra M, Prates PR, et al. Effects of therapeutic angiogenesis with plasmid VEGF165 on ventricular function in a canine model of chronic myocardial infarction. Rev Bras Cir Cardiovasc. 2009;24(2):143-9. [MedLine] View article
Article receive on Thursday, February 11, 2010






 All scientific articles published at bjcvs.org are licensed under a Creative Commons license
All scientific articles published at bjcvs.org are licensed under a Creative Commons license