Cristiane Delgado Alves RodriguesI; Marcos Mello MoreiraII; Núbia Maria Freire Vieira LimaII; Luciana Castilho de FigueirêdoII; Antônio Luis Eiras FalcãoIII; Orlando Petrucci JuniorIII; Desanka DragosavacII
DOI: 10.5935/1678-9741.20140103
APACHE II: Acute Physiology and Chronic Health Evaluation II
ALI: Acute Lung Injury
ARDS: Acute Respiratory Distress Syndrome
CH: Clinics Hospital
CPB: Cardiopulmonary Bypass
FiO2: Fraction of inspired oxygen
ICU: Intensive Care Unit
PaO2: Partial pressure of oxygen
PCWP: Pulmonary capillary wedge pressure
PEEP: Positive end-expiratory pressure
SD: Standard deviation
SIRS: Systemic inflammatory response syndrome
TDGE: Transient Dysfunction of Gas Exchange
UNICAMP: Universidade Estadual de Campinas
VAP: Ventilator-Associated Pneumonia
INTRODUCTION
Cardiac surgery has a direct influence on the respiratory system in patients with heart disease, affecting the morbidity and mortality of patients postoperatively. Respiratory complications after cardiac surgery have been described in the literature since 1965[1]. Respiratory system dysfunction can occur due to general anesthesia, median sternotomy (which leads to instability of the upper chest), cardiopulmonary bypass (CPB), prolonged myocardial ischemia, manipulation during surgery, and number of chest tubes[1-4]. Changes in pulmonary function occurring in the postoperative period of cardiac surgery with CPB are secondary to the reaction of heparin with protamine complex, edema, congestion, and lung damage, in addition to microatelectasis. In most cases, there is an absence of mechanical ventilation during CPB, which, coupled with the inflammatory response due to surgical trauma, leads to changes in respiratory mechanics[2,5].
During CPB, blood contact occurs with non-endothelial surfaces, leading to blood clotting; this clotting occurs along with the activation of the inflammatory cascades and contributes to the increased weight of the pulmonary parenchyma and the additional breakdown of cellular units, further impairing gas exchange in these patients. Thus, the presence of postoperative hypoxemia is secondary to all these changes that impair the ventilation/perfusion ratio[6]. Due to the occurrence of transient dysfunction of gas exchange (called TDGE in this study) after surgery, the terms acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) have been widely used in patients after cardiac surgery with CPB in recent decades. According to the criteria of the first consensus definitions of ARDS and ALI (1994), several studies reported the incidence of these disorders after such a procedure[4].
Recently, the criteria for the diagnosis of ARDS have been changed. The current definition is based on the degree of hypoxemia, represented by the partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2), namely, mild ARDS (PaO2/FiO2 between 200 mmHg and 300 mmHg), moderate ARDS (PaO2/FiO2 between 100 mmHg and 200 mmHg), and severe ARDS (PaO2/FiO2<100 mmHg). In addition, four factors were included for the diagnosis of severe ARDS (refractory hypoxemia, radiographic severity, respiratory system compliance, and positive end-expiratory pressure)[7].
The criteria for defining DTGE this study were the same ones used in the latest rankings of ARDS, described above. Therefore, the primary objective of the present study was to evaluate the presence of TDGE after cardiac surgery and to determine if there is an association between post-cardiac surgery TDGE and cardiorespiratory events. As a secondary objective, the presence of risk factors for the development of cardiorespiratory complications in the postoperative period was evaluated.
METHODS
Study design and ethical considerations
The research project was approved by the ethics committee of the institution, under the assigned number 409,460/2013. During the research, the medical information and the privacy of the patients were kept confidential. The data from this study were obtained from the database and charts of patients from the Clinics Hospital (CH) of the State University of Campinas (UNICAMP).
Population
Consecutive patients undergoing cardiac surgery and cardiac procedures referred to the ICU in between June 2007 and November 2011 were included in this study. The patients included males and females and over the age of 14 years. The initial observed sample included 942 patients, whose data were collected prospectively and consecutively and stored in the database of the ICU of Clinics Hospital at UNICAMP. Data collection was performed daily, at the bedside by specially trained professionals (1998/3432 Ordinance). The data from patients who had been readmitted and the data from patients with incomplete medical records were excluded from the statistical analysis.
The demographic and clinical variables assessed in this study were as follows:
1) type of surgery or cardiac procedure performed;
2) occurrence of TDGE after surgery and cardiac procedures. TDGE represents changes in PaO2/FiO2 present up to 48 hours after surgery;
3) preoperative cardiorespiratory system background and its association with postoperative TDGE type;
4) presence of complications, infections, and post-operative interventions and their associations with postoperative TDGE type;
5) length of stay in the ICU, occurrence of death in the ICU, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, mortality provided by APACHE II, and their association with TDGE;
6) risk factors for developing cardiorespiratory complications in the postoperative period.
Measuring instruments
The APACHE II scores were calculated for the patient's first 24 hours in the ICU; the patients' mortality figures were provided by that score[8].
Calculation of the ratio PaO2/FiO2
Tracks of the current classification for defining ARDS were employed to determine four patient groups with study TDGE: 1) absence of TDGE (PaO2/FiO2>300 mmHg), 2) mild TDGE (PaO2/FiO2 between 200 mmHg and 300 mmHg), 3) moderate TDGE (PaO2/FiO2 between 100 mmHg and 200 mmHg), and 4) severe TDGE (PaO2/FiO2<100 mmHg). The values of PaO2/FiO2 were from the arterial blood gas analysis on the first postoperative day, i.e., up to the 48th hour in the ICU.
ICU protocols and procedures in the surgical center
The heart surgeries and procedures were carried out by the same team for all patients. Surgical techniques and procedures complied with the standards described by the work-processes manuals of CH/UNICAMP. At the end of the surgery or termination of the procedure, patients presenting favorable clinical conditions were extubated in the surgical room. If the patient's clinical conditions were not favorable, they were referred to the postoperative ICU, intubated, and mechanically ventilated. Postoperative ICU patients were admitted by the multidisciplinary team and were then processed using the admission protocol for surgical patients in the ICU. The patients were then under the care of the ICU staff, who attended to the patients according to the specific protocols of the unit.
Phases of the retrospective study
This study consisted of a data collection period followed by the assessment and review of the database and the medical records of patients. The tabulation of the data was revised, and finally, the statistical analysis was performed. For the statistical analysis, the program SAS System for Windows, version 9.2 was used. Continuous variables are presented as the mean ± SD. Categorical variables are expressed as absolute values and percentages. To verify an association, the chi-square test or Fisher's exact test was used when necessary. To test the relationship between the disease preoperatively and TDGE, as well as the relationship between complications and interventions in the ICU and TDGE, the logistic regression model with proportional odds was used. ANOVA by ranks with transformation followed by the Tukey test was used to compare TDGE groups (no, mild, moderate, severe) with respect to the length of hospitalization in intensive care, APACHE II and predicted mortality. To verify a linear trend in proportions, the Cochran-Armitage test was applied. We used Cox regression analysis to identify factors associated with univariate complications and death. P<0.05 was considered significant for all tests.
RESULTS
Of the initial sample, 717 patients fulfilled the criteria of the study. Patients who were readmitted or had data missing from their charts were excluded from the study. There were 442 (61.6%) male and 275 (38.4%) female patients. The average age of the patients was 56.1 years (SD=13.7). The types of surgery and cardiac procedures performed are described in Table 1.
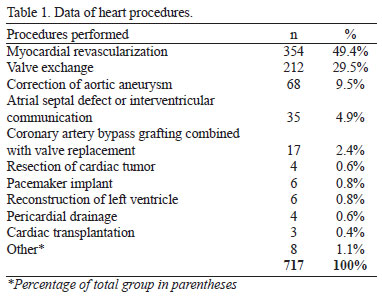
In Table 2, the frequencies for TDGE are presented.

In Table 3, the background and preoperative comorbidities and their correlations with postoperative TDGE are stated.
TDGE was associated with age (P<0.0001), and age was also a risk factor (P<0.0001) for TDGE, as shown in Table 4.
Interventions and complications in the ICU and their association with the appearance of TDGE are described in Table 5.
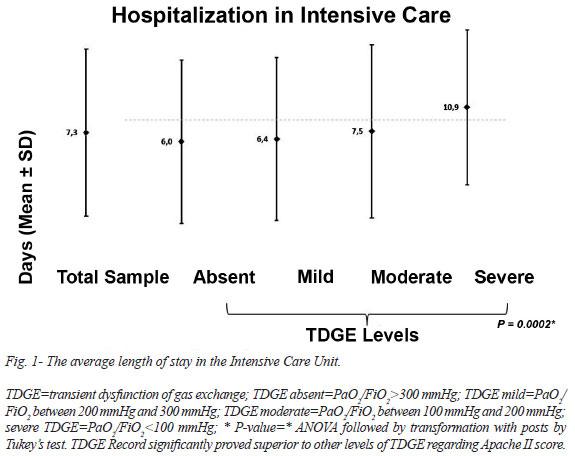
The occurrence of death within 48 hours and after 48 hours are described in Table 6. The he average length of stay in the ICU and the mortality predicted by APACHE II are described in Figure 1 and Figure 2.
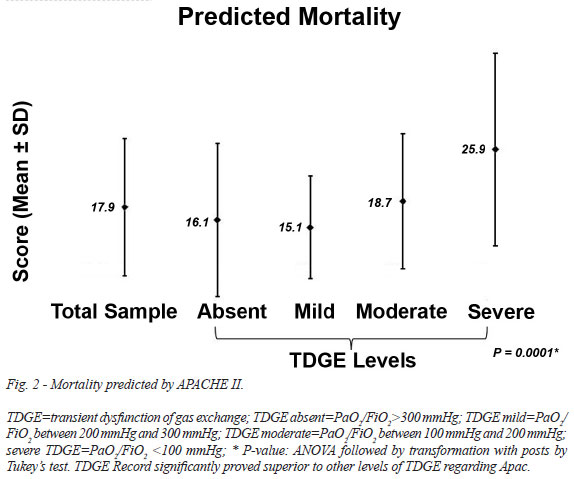
Death within the first 48 hours after surgery and death in the ICU were associated with the APACHE II score (P=0.0105 and P<0.0001, respectively). Death within the first 48 hours after surgery and death in the ICU were both associated with predicted mortality (P=0.0001, and P<0.0114, respectively).
DISCUSSION
Demographic variables, background, and preoperative comorbidities
The majority of the study population was male (61.6%), with an average age of 56.1 years. This finding is consistent with reports that the male population has a higher surgical incidence (70.1%) than females in the under-60 population[9]. In this study, myocardial revascularization surgery was the most common (49.4%), corroborating the findings of Laizo et al.[10]. The preoperative comorbidities diabetes mellitus (19.5%) and hypertension (52.6%) were prevalent. A previous study observed diabetes mellitus in 29.6% of cases[8]. Compared with other developed countries, Brazil has a higher incidence of hypertension (90.7% vs. 60%) and diabetes mellitus (37.2% vs. 29%)[9]. Diseases such as hypertension, autoimmune diseases, peripheral vascular disorders, and metabolic syndrome must be controlled and require great care in the immediate postoperative period[10].
Other studies report preoperative comorbidities of dyslipidemia in 48% of cases and a family background of coronary artery disease in 38% of cases but report hypertension in 75%[11] to 79%[2] of patients. In developed countries, cardiovascular diseases are the leading causes of death and are increasing in occurrence in developing countries[12]. Oliveira et al.[11] measured morbidity by measuring the occurrence of the postoperative complications described above and the mortality as the number of deaths. In our study, 0.84% of individuals died within 48 hours, and 9.21% died after 48 hours of intensive care.
In Brazil, the mortality rate for myocardial revascularization is 6.2%. Several postoperative management protocols of cardiac surgery have been studied with the purpose of predicting mortality[10]. In the study of Ribeiro et al.[13], especially in the postoperative period of cardiac surgery, 115,021 pulmonary complications contributed to the overall mortality rate of 8%. In Brazil, the mortality rate after cardiovascular surgery is approximately 8% in the Unified Health System[14]. APACHE II is an index that classifies critical patients according to the severity of their condition[15]. Our patients had high APACHE II scores, similar to the study by Feijó et al.[16]. In a prospective trial of 520 patients lasting 13 months in the ICU of Hospital Sao Paulo, in which the APACHE II prognostic index was applied, scores > 25 were associated with a higher risk of death[15]. In our study, the APACHE II score (12.8 ± 4.2 points) and predicted mortality (17.9±9.5 points) were correlated with the occurrence of severe TDGE (P=0.0001 for both), demonstrating that APACHE II score was effective to determine the severity of the patients in this study. Although mortality was high (10.5%), it was less than expected.
Occurrence of TDGE after surgery and cardiac procedures
Respiratory failure after heart surgery is an important factor of postoperative morbidity and mortality[4]. In this study, 15 (2%) patients had ARDS, 27.7% mild TDGE, 56.1% moderate TDGE, and 5.4% severe TDGE. In a study by Szeles et al.[5], the PaO2/FiO2 ratio was used to assess the impact of the seriousness of the hypoxemia in the immediate postoperative period. The patients were divided into three groups: PaO2/FiO2>200 (45.8%); PaO2/FiO2 between 150 and 200 (26.9%) and PaO2/FiO2<150 (27.3%). That study reported that transient hypoxemia was not affected by an increase of mechanical ventilation time and that the inflammatory response to surgical trauma and CBP caused lung damage, explaining the transitional hypoxemia[5].
ARDS was present in 0.4% to 1.32% of the patients[4]. ARDS was first described by Ashbaugh & Petty, in 1967, with these common characteristics: tachypnea, hypoxemia, persistent opacification on chest X-ray, decreased complacency, and high mortality. In 1994, the first consensus for ARDS was published, which defined four aspects: acute presentation, presence of hypoxemia (PaO2/FiO2<200 mmHg), infiltrated bilateral on chest X-ray, and pulmonary capillary wedge pressure (PCWP) less than 18 cmH2O (to rule out heart failure). Also defined in this consensus, similar to ARDS, was ALI, defined as being present when the PaO2/FiO2 ratio is between 200 and 300 mmHg[17].
Over the years, the criteria for ARDS did not correspond to the manifestations of the syndrome. In 2012, the criteria were revised using the following classification: the term acute was defined as the occurrence of events of manifestation in one week or less; the term ALI was abandoned; and the measurement of the PaO2/FiO2 relationship was amended to require a minimal amount of positive end-expiratory pressure (PEEP)[7]. In addition, three categories of ARDS were proposed: mild, moderate, and severe, based on the PaO2/FiO2 ratio; chest X-ray criteria were clarified to improve reliability between examiners; and the PCWP criterion was removed and clarity was added to improve the ability to rule out cardiac causes of bilateral infiltrates[17]. The last definition includes the measurement of PEEP, limiting the possibilities of diagnosis for patients without mechanical ventilation, but the diagnosis of ARDS does not exclude these patients.
To evaluate our patients under the new classification of severity of the ARDS PaO2/FiO2 ratio, we found that 89.26% fit this criterion for diagnosis, suggesting a high incidence of ARDS in this population. However, using only the PaO2/FiO2 ratio is not sufficient to define the presence of ARDS. Other factors are included in the dysfunctional gas exchange in the postoperative period of cardiac surgery, such as systemic inflammatory response syndrome (SIRS) with pulmonary repercussion, hyperdynamic frame, interstitial pulmonary edema, microatelectasis, and reduced surfactant. In most cases, the pulmonary radiological image is normal, undercommitted lung compliance, and patients recover from this dysfunction in a few hours, including extubation after a few hours in intensive care.
In the present study, of 89.26% of patients who presented with TDGE, only 2% evolved with ARDS, according to the criteria of the last consensus. The classification of ARDS as mild, moderate, and severe was used to determine the degree of TDGE. Based on these findings, the term ARDS should only be used for patients who meet all the diagnostic criteria. Therefore, we suggest that this acute and transient hypoxia that occurs in the postoperative period of cardiac surgery (within 48 hours) be called transient dysfunction of gas exchange, as we have in this work.
Association of TDGE with preoperative and postoperative factors
In our sample, increasing age was associated with increasing severity of TDGE, especially in patients older than 75 years, whose risk is three times higher than patients younger than 44 years. In the study of Oliveira et al.[9], age>70 years was significantly associated with greater mortality (P<0.002). In this study, the average length of stay in the ICU was 7.3±11.9 days, similar to the average of 4.16±3.76 days in the study by Laizo et al.[10]. There was a tendency for the prevalence of severe TDGE to increase from 2007 to 2011(P=0.01).
Hypertension and cardiogenic shock were associated with the emergence of moderate TDGE postoperatively (P=0.022 and P=0.019, respectively) and were risk factors (P=0.001 and P=0.002, respectively) for the development of this dysfunction. Diabetes mellitus was a risk factor for TDGE (P=0.035). Diabetic patients have chronic vasculitis, and when associated with the postoperative inflammatory process, this condition worsens, altering the relative pulmonary ventilation/perfusion ratio, which corresponds to changes in gas exchange.
The postoperative complications influence the length of hospitalization of the patient, generating increased costs and hospital mortality[18]. In this study, pneumonia was present in 8.9% of cases and was associated with moderate TDGE (P=0.001), which was a risk factor (P=0.0005) for the occurrence of postoperative pneumonia. Ventilator-associated pneumonia (VAP) was present in 0.6% of the cases and was associated with severe TDGE (P=0.042), which in turn was a risk factor (P=0.003) for the development of VAP.
The low incidence of VAP in the study may be explained by the implementation of prophylactic measures and the awareness of the professionals of the ICU at the beginning of 2007. In a previous study, lung infection was more common among the infectious complications (15.3%)[19]. In another study, 7332 patients undergoing cardiac surgery, infectious outbreaks were identified in 29 patients, 55% of them primary infections[14]. However, there is no study reporting the occurrence of postoperative pulmonary complications[20].
Oliveira et al.[11] indicated the following as predictors of postoperative infections: body mass index > 40 kg/m2, preoperative hemodialysis, cardiogenic shock, preoperative age > 85 years, preoperative immunosuppressive treatment, diabetes mellitus, CPB > 200 minutes, and the revascularization of three or more vessels.
Among the complications encountered in the period after surgery, those that occur in the respiratory system contribute significantly to morbidity and mortality related to cardiac surgery, as 3.5% to 10% of these morbidities and mortalities are caused by respiratory complications[21]. In large part, these complications are explained by the use of CPB, which causes an increase of inflammatory mediators leading to decreased ventricular contractility, which consequently increases vascular permeability and resistance of the organs.
Specifically in the pulmonary circulation, inflammatory fluid accumulates interstitially, leading to the formation of microatelectasis, hypoxemia, and hypoxic vasoconstriction. These conditions decrease the local production of pulmonary surfactant, which leads to worsening pressures, lung collapse, and pulmonary dysfunction, generating losses in respiratory mechanics and increased respiratory work[12,22]. This situation results in pneumonic complications in respiratory mechanics[23]. Morsch et al.[2] used pulmonary radiological changes as diagnostic criteria for unventilated areas and/or consolidation, pleural effusion, and collapsed lung. Diagnostic criteria of postoperative lung infections are not standard in the scientific literature and are associated or not with radiological changes, leukocytosis, body hyperthermia, and isolation of pathogens in culture or in microscopic analysis of[21].
Hypoxemia is among the leading pulmonary complications in the postoperative period of cardiac surgery; however, other complications, such as pleural effusion, pneumonia, pneumothorax, reintubation, and ventilatory insufficiency, are also observed[24]. We used non-invasive mechanical ventilation therapeutically in 2.1% of cases, reintubation in 9.6%, and tracheostomy in 3.6%. Reintubation was a risk factor (P=0.042) for only postoperative TDGE.
Severe TDGE was associated with patients who had renal replacement therapy (P=0.0005), hemotherapy (P=0.0001), and cardiac arrhythmia (P=0.0450), with respective risk factors 2.34, 1.72, and 1.79. A large amount of information in this study was collected from patient records, so the loss of information is inexcusable. We sorted the information by category to extract the largest amount of available information.
CONCLUSION
We concluded that TDGE, in varying degrees, was present at the time of surgery and during postoperative cardiac procedures. Preoperative hypertension and cardiogenic shock were associated with the occurrence of postoperative TDGE. The preoperative risk factors included hypertension, cardiogenic shock, and diabetes. Postoperatively, pneumonia, VAP, renal replacement therapy, hemotherapy, and cardiac arrhythmia were associated with the appearance of a certain degree of TDGE, which was a risk factor for reintubation, pneumonia, VAP, and renal replacement therapy during the postoperative period from cardiac surgery and during cardiac procedures.
ACKNOWLEDGMENTS
The authors acknowledge the work done by the Office of Biostatistics, School of Medical Sciences, State University of Campinas. In particular, we would like to thank Cleide Aparecida Moreira Silva for his dedication to this work.
We also appreciate nurse Claudinéia Muterle Logato Marmirolli for collecting data in the Intensive Care Unit of the Hospital of the State University of Campinas.
REFERENCES
1. Guizilini S, Gomes WJ, Faresin SM, Carvalho ACC, Jaramillo JI, Alves FA, et al. Effects of the pleural drain site on the pulmonary function after coronary artery bypass grafting. Rev Bras Cir Cardiovasc. 2004;19(1):47-54. View article
2. Morsch KT, Leguisamo CP, Camargo MD, Coronel CC, Mattos W, Ortiz LDN, et al. Perfil ventilatório dos pacientes submetidos a cirurgia de revascularização do miocárdio. Rev Bras Cir Cardiovasc. 2009;24(2):180-7. [MedLine] View article
3. Arcêncio L, Souza MD, Bortolin BS, Fernandes ACM, Rodrigues AJ, Évora PRB. Cuidados pré e pós-operatórios em cirurgia cardiotorácica: uma abordagem fisioterapêutica. Rev Bras Cir Cardiovasc. 2008;23(3):400-10. [MedLine] View article
4. Rodrigues CDA, Oliveira RARA, Soares SMTP, Figueiredo LC, Araújo S, Dragosavac D. Lesão pulmonar e ventilação mecânica em cirurgia cardíaca: revisão. Rev Bras Ter Intensiva. 2010;22(4):375-83.
5. Szeles TF, Yoshinaga EM, Alencar W, Brudniewski M, Ferreira FS, Auler Jr JOC, et al. Hipoxemia após revascularização miocárdica: análise dos fatores de risco. Rev Bras Anestesiol. 2008;58(2):124-36. [MedLine]
6. Padovani C, Cavenaghi OM. Recrutamento alveolar em pacientes no pós-operatório imediato de cirurgia cardíaca. Rev Bras Cir Cardiovasc. 2011;26(1):116-21. [MedLine] View article
7. ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-33. [MedLine]
8. Ledur P, Almeida L, Pellanda LC, Schaan BD. Preditores de infecção no pós-operatório de cirurgia de revascularização miocárdica. Rev Bras Cir Cardiovasc. 2011;26(2):190-6. [MedLine] View article
9. Oliveira EL, Westphal GA, Mastroeni MF. Características clínico-demográficas de pacientes submetidos à cirurgia de revascularização do miocárdio e sua relação com a mortalidade. Rev Bras Cir Cardiovasc. 2012;27(1):52-60. [MedLine] View article
10. Laizo A, Delgado FEF, Rocha GM. Complicações que aumentam o tempo de permanência na unidade de terapia intensiva na cirurgia cardíaca. Rev Bras Cir Cardiovasc. 2010;25(2):166-71. [MedLine] View article
11. Oliveira DC, Oliveira Filho JB, Silva RF, Moura SS, Silva DJ, Egito EST, et al. Sepse no pós-operatório de cirurgia cardíaca: descrição do problema. Arq Bras Cardiol. 2010;94(3):332-6.
12. Renault JA, Costa-Val R, Rossetti MB. Fisioterapia respiratória na disfunção pulmonar pós-cirurgia cardíaca. Rev Bras Cir Cardiovasc. 2008;23(4):562-9. [MedLine] View article
13. Ribeiro AL, Gagliardi SP, Nogueira JL, Silveira LM, Colosimo EA, Lopes do Nascimento CA. Mortality related to cardiac surgery in Brazil, 2000-2003. J Thorac Cardiovasc Surg. 2006;131(4):907-9. [MedLine]
14. Gomes WJ, Mendonça JT, Braile DM. Resultados em cirurgia cardiovascular: oportunidade para rediscutir o atendimento médico e cardiológico no sistema público de saúde do país. Rev Bras Cir Cardiovasc. 2007;22(4):III-VI. [MedLine]
15. Costa JI, Gomes do Amaral JL, Munechika M, Juliano Y, Bezerra-Filho JG. Severity and prognosis in intensive care: prospective application of the APACHE II index. Sao Paulo Med J. 1999;117(5):205-14. [MedLine]
16. Feijó CAR, Leite Júnior FO, Martins ACS, Furtado Júnior AH, Cruz LLS, Meneses FA. Gravidade dos pacientes admitidos à unidade de terapia intensiva de um hospital universitário brasileiro. Rev Bras Ter Intensiva. 2006;18(1):18-21. [MedLine]
17. Angus DC. The acute respiratory distress syndrome: what's in a name? JAMA. 2012;307(23):2542-4. [MedLine]
18. Piotto RF, Ferreira FB, Colósimo FC, Silva GS, Sousa AG, Braile DM. Fatores preditores independentes de ventilação mecânica prolongada em pacientes submetidos à cirurgia de revascularização miocárdica. Rev Bras Cir Cardiovasc. 2012;27(4):520-8. [MedLine] View article
19. Strabelli TMV, Stolf NAG, Uip DE. Uso prático de um índice de risco de complicações após cirurgia cardíaca. Arq Bras Cardiol. 2008;91(5):342-7. [MedLine]
20. Westerdahl E, Lindmark B, Almgren SO, Tenling A. Chest physiotherapy after coronary artery bypass graft surgery: a comparison of three different deep breathing techniques. J Rehabil Med. 2001;33(2):79-84. [MedLine]
21. Brasher PA, McClelland KH, Denehy L, Story I. Does removal of deep breathing exercises from a physiotherapy program including pre-operative education and early mobilisation after cardiac surgery alter patient outcomes? Aust J Physiother. 2003;49(3):165-73. [MedLine]
22. Barbosa RAG, Carmona MJC. Avaliação da função pulmonar em pacientes submetidos à cirurgia cardíaca com circulação extracorpórea. Rev Bras Anestesiol. 2002;52(6):689-99. [MedLine]
23. Taylor GJ, Mikell FL, Moses HW, Dove JT, Katholi RE, Malik SA, et al. Determinants of hospital charges for coronary artery bypass surgery: the economic consequences of postoperative complications. Am J Cardiol. 1990;65(5):309-13. [MedLine]
24. Ferreira LL, Marino LHC, Cavenaghi S. Fisioterapia cardiorrespiratória no paciente cardiopata. Rev Bras Clin Med. 2012;10(2):127-31.
No financial support.
Authors' roles & responsibilities
CDAR: Analysis and/or interpretation of data, final approval of the manuscript, study design, manuscript writing or critical review of its content
MMM: Final approval of the manuscript
NMFL: Analysis and/or interpretation of data, statistical analysis, final approval of the manuscript, study design, manuscript writing or critical review of its content
LCF: Final approval of the manuscript
ALEF: Final approval of the manuscript
OPJ: Final approval of the manuscript
DD: Analysis and/or interpretation of data, final approval of the manuscript, study design, manuscript writing or critical review of its content
Article receive on Saturday, December 28, 2013
 All scientific articles published at bjcvs.org are licensed under a Creative Commons license
All scientific articles published at bjcvs.org are licensed under a Creative Commons license