INTRODUCTION
The prognosis and life expectancy of individuals with aortic aneurisms depends on several factors, including the time of diagnosis, the adequate choice of therapeutic modality, as well as the postoperative radiological follow up.
For many years, the most indicated surgical treatment for aortic aneurisms was the substitution of the dilated segment using a prosthetic material, with or without the re-implantation of related arterial branches [1].
In 1991, the success obtained by Parodi et al. in the correction of an abdominal aortic aneurism using an endoprosthesis, gave vascular and cardiovascular surgeons another option of a novel and promising therapy, in particular for individuals with numerous or considerably deleterious comorbidities [2]. Despite of the satisfactory results obtained with the endovascular treatment of aortic aneurisms, some complications have been documented, such as the occurrence of strokes, paraplegia, prosthetic migration, embolic phenomenon and leaks [3-6].
Gabriel et al. [7] recently demonstrated that the endovascular procedure for the correction of aortic aneurisms involves factors related to a systemic inflammatory response, both in the acute phase and in a later phase of the postoperative follow up. These authors postulated that inflammatory mediators, such as cytokines and cellular adhesion molecules, can be better evaluated by means of curves that register the variability of their serum levels over time. This knowledge is of notable relevance for the professional that performs the endovascular procedure, largely because it enables the correlation of different postoperative clinical manifestations with the inflammatory role that each marker may play in the organism. Moreover, by knowing and understanding this correlation, it will be possible, if necessary, to institute an effective medicinal treatment.
However, there are no studies that define which inflammatory variables are elementary to the prognosis of individuals submitted to the endovascular treatment of aortic aneurisms [7].
Hence, the aim of this study is to propose an inflammatory risk score for individuals submitted to the endovascular correction of aortic aneurisms.
METHODS
This research was approved by the Research Ethics Committee of the Federal University of Sao Paulo (UNIFESP) and complies with the Declaration of Helsinki. All the individuals signed written consent forms before participating in the study.
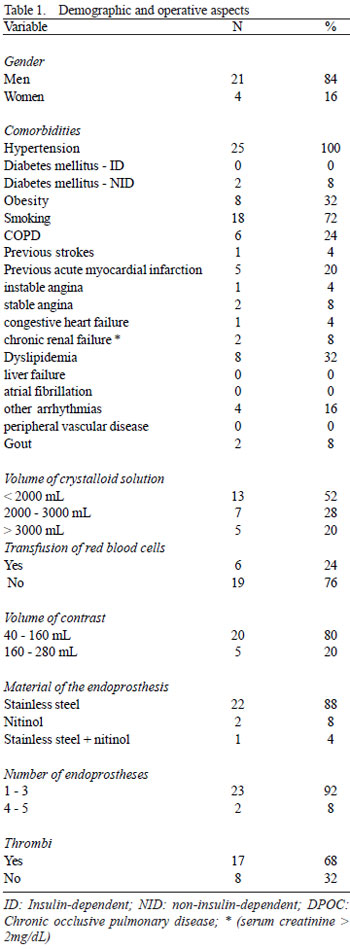
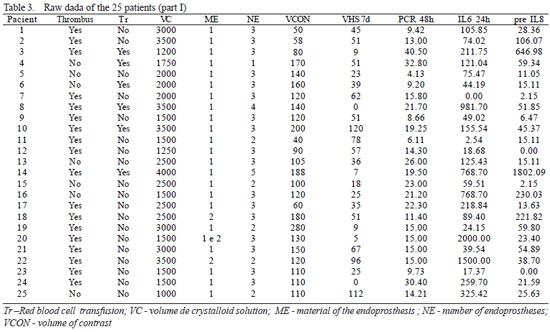
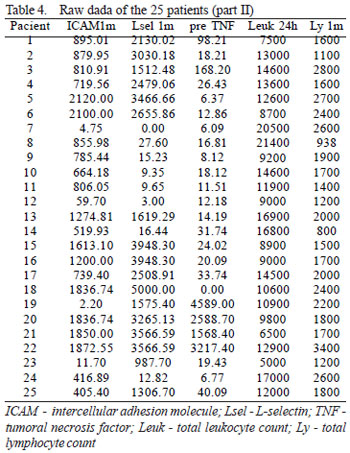
In the period from March to December 2005, 25 patients with thoracic or abdominal aortic aneurisms were prospectively enrolled. The exclusion criteria were acute dissections, traumatic dissections, inflammatory aneurisms, conjunctive tissue diseases and patients presenting with any sign of infection or immunodeficiency at the time of the endovascular procedure. The demographic characteristics and operative aspects are shown in Table 1. Some inflammatory variables considered in this study were evaluated in the preoperative period (24 hours before the endovascular procedure) and at several intervals during the postoperative follow up (1 hour, 6 hours, 24 hours, 48 hours, 7 days, 1 month, 2 months and 3 months). All these variables were considered parametric except for intramural thrombi and material of the endoprosthesis, which were analyzed as non-parametric. The parametric variables were employed to obtain a risk score model, considering only the time at which they were most prevalent (Table 2), based on the study performed by Gabriel et al. [7]. The hypothesis was, that the higher the values of the parametric variables, the greater the risk of inflammation; on the other hand, the simple presence of non-parametric variables would be compatible with a greater probability of risk. The raw data of 25 patients in relation to the variables and intervals of time of greatest prevalence, as demonstrated in Tables 3 and 4, allows us to apply a step-by-step method to configure a risk score.
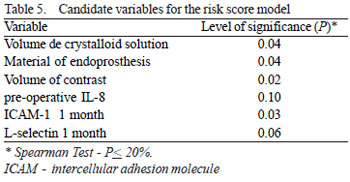
Initially, the Spearman test determined which inflammatory variables can be classified as candidates for the score model based on a level of significance d" 20% (Table 5). Subsequently, logistic regression was applied to select variables that effectively would be used in the establishment of an inflammatory risk score. In this final selection phase, a level of significance < 10% was adopted. Analysis of the ROC curve was utilized to determine cut-off values for the variables identified by the logistic regression test.
RESULTS
The logistic regression test identified the volume of crystalloid solution and the preoperative level of IL-8 as possible variables for the formation of an inflammatory risk score. The analysis of the ROC curve (Figure 1) indicated that the highest values of sensitivity and specificity found for the volume of crystalloid solution and the preoperative IL-8 level were 1850 mL and 33.53 pg/mL, respectively and, thus, these should be the cut-off values for the variables.
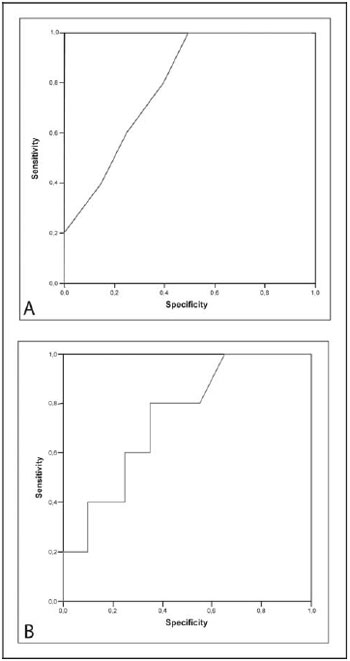
Fig. 1 - A: Analysis of the ROC curve for the volume of crystalloid solution; B: Analysis of the ROC curve for the pre-operative values of IL-8
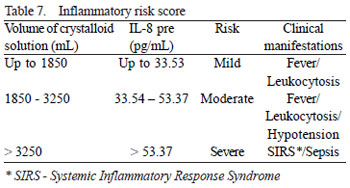
Hence, it was possible to allocate the values of both variables, found for the 25 patients, to different categories of inflammatory risk, as well as to estimate the maximum probability of risk (Table 6). Table 7 demonstrates the model of inflammatory risk score of aortic endoprostheses based on the correlation of the cut-off values and different numeric intervals with the most common clinical manifestations.
DISCUSSION
The treatment of aortic aneurisms by open surgery with the associated complications results, in some circumstances, in a high risk of morbidity and mortality. Added to this is the fact that repair of this disease by the conventional approach requires, in most cases, the use of cardiopulmonary bypasses, which confers a greater risk of postoperative complications.
The viability and efficacy of endovascular therapy in the correction of aortic aneurisms have been consolidated due to the possibility of performing less invasive procedures, in particular when there are associated risk factors, such as age, chronic obstructive pulmonary disease, hypertension, smoking, chronic renal failure and cardiovascular diseases [8-10].
The complex reactions resulting from the interaction between the aortic endothelium and the endoprosthesis can be conceptualized as post-implantation syndrome, which is characterized by the expression of multiple and, often, lethal organic effects. Some subjacent events are being studied in order to clarify the main mechanisms that cause this syndrome. Extensive endothelial activation after the implantation of an endoprosthesis, activation due to the contact of blood components with the surface of the endoprosthesis, as well as ischemia-reperfusion lesions owing to prolonged periods of clamping of the femoral arteries, have been considered possible etiologies of this process. Additionally, from the physiopathological point of view, some clinical manifestations may be correlated to these events including leukocytosis, fever and coagulation disorders.
An estimation of inflammatory risk in the endovascular treatment of aortic aneurisms is still not possible due to the inexistence of a specific score that encompasses the inflammatory variables inherent to this type of therapy. In this study, the follow up of 25 patients submitted to the endovascular treatment demonstrates that there are several inflammatory variables involved in the systemic inflammatory response induced by contact of the endothelium with the endoprostheses; however, the volume of crystalloid solution and the preoperative value of IL-8, in particular, were identified as probable variables to be included in the creation of an original model of an inflammatory risk score.
Several mechanisms are involved in the inflammatory response induced by the infusion of crystalloid solution. A number of authors have postulated that, the more crystalloid solution utilized, the greater the transcription of pro-inflammatory genes, the greater the expression of cellular adhesion molecules, such as E-selectin and P-selectin, the greater the neutrophil activation and the greater the release of cytokines and hydrogenated metabolites will be [11-15]. Pascual et al. [16,17] demonstrated that the infusion of hypertonic solutions might reduce the adhesion and transmigration of inflammatory cells.
The majority of aortic aneurisms are primarily associated with the atherosclerotic process and, secondly, correlated with transmural degenerative processes, neovascularization, reduction in the quantity of smooth muscle cells and chronic inflammatory infiltration, The dominant cells of this infiltration are T helper 2 lymphocytes, that participate in the synthesis of inflammatory mediators such as IL-8 and TNF-a. Lindeman et al. [18] removed fragments of the aneurismal walls from the human aorta and demonstrated that, in this type of tissue, there is increased expression and activation of interleukins, in particular IL-6 and IL-8. These findings are compatible with the relevance of preoperative values of IL-8 identified in the current study, which is fundamental to an adequate estimation of inflammatory risk of aortic endoprostheses [18-21].
Although the inflammatory risk score for aortic endoprostheses is useful in obtaining better postoperative results, its applicability should not discard the impact on the cost of the procedure and, thus, attention should be paid to this question.
CONCLUSION
The authors of the current study propose a model of an inflammatory risk score for the endovascular treatment of aortic aneurisms, in an attempt to determine which inflammatory factors effectively might have a reasonable impact in prognostic terms. In the first analysis, the volume of crystalloid solution utilized in the endovascular procedure and the pre-operative value of IL-8 have been indicated for this evaluation. Nevertheless, this investigation must be amplified with further studies to optimize the results.
REFERENCES
1
1. Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008;85(1 Suppl):S1-41. [
MedLine]
2. Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5(6):491-9. [
MedLine]
3. Tehrani HY, Peterson BG, Katariya K, Morasch MD, Stevens R, DiLuozzo G, et al. Endovascular repair of thoracic aortic tears. Ann Thorac Surg. 2006;82(3):873-7.
4. Iannelli G, Piscione F, Di Tommaso L, Monaco M, Chiariello M, Spampinato N. Thoracic aortic emergencies: impact of endovascular surgery. Ann Thorac Surg. 2004;77(2):591-6. [
MedLine]
5. Koning GG, Vallabhneni SR, Van Marrewijk CJ, Leurs LJ, Laheij RJF, Buth J. Procedure-related mortality of endovascular abdominal aortic aneurysm repair using revised reporting standards. Rev Bras Cir Cardiovasc. 2007;22(1):7-14.
6. Saadi EK, Gastaldo F, Dussin LH, Zago AJ, Barbosa G, Moura L. Tratamento endovascular dos aneurismas de aorta abdominal: experiência inicial e resultados a curto e médio prazo. Rev Bras Cir Cardiovasc. 2006;21(2):211-6.
7. Gabriel EA, Locali RF, Romano CC, Duarte AJ, Palma JH, Buffolo E. Analysis of the inflammatory response in endovascular treatment of aortic aneurysms. Eur J Cardiothorac Surg. 2007;31(3):406-12. [
MedLine]
8. Juvonen T, Ergin MA, Galla JD, Lansman SL, Nguyen KH, McCullough JN, et al. Prospective study of the natural history of thoracic aortic aneurysms. Ann Thorac Surg. 1997;63(6):1533-45. [
MedLine]
9. Griepp RB, Ergin MA, Galla JD, Lansman SL, McCullough JN, Nguyen KH, et al. Natural history of descending thoracic and thoracoabdominal aneurysms. Ann Thorac Surg. 1999;67(6):1927-30.
10. Strachan DP. Predictors of death from aortic aneurysm among middle-aged men: the Whitehall study. Br J Surg. 1991;78(4):401-4. [
MedLine]
11. Alam HB, Sun L, Ruff P, Austin B, Burris D, Rhee P. E - and Pselectin expression depends on the resuscitation fluid used in hemorrhaged rats. J Surg Res. 2000;94(2):145-52. [
MedLine]
12. Alam HB, Stanton K, Koustova E, Burris D, Rich N, Rhee P. Effect of different resuscitation strategies on neutrophil activation in a swine model of hemorrhagic shock. Resuscitation. 2004;60(1):91-9. [
MedLine]
13. Watters JM, Tieu BH, Todd SR, Jackson T, Muller PJ, Malinoski D, et al. Fluid resuscitation increases inflammatory gene transcription after traumatic injury. J Trauma. 2006;61(2):300-8.
14. Zhang H, Voglis S, Kim CH, Slutsky AS. Effects of albumin and Ringer's lactate on production of lung cytokines and hydrogen peroxide after resuscitated hemorrhage and endotoxemia in rats. Crit Care Med. 2003;31(5):1515-22. [
MedLine]
15. Sun LL, Ruff P, Austin B, Deb S, Martin B, Burris D, et al. Early up-regulation of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 expression in rats with hemorrhagic shock and resuscitation. Shock. 1999;11(6):416-22. [
MedLine]
16. Pascual JL, Khwaja KA, Ferri LE, Giannias B, Evans DC, Razek T, et al. Hypertonic saline resuscitation attenuates neutrophil lung sequestration and transmigration by diminishing leukocyteendothelial interactions in a two-hit model of hemorrhagic shock and infection. J Trauma. 2003;54(1):121-30.
17. Pascual JL, Ferri LE, Seely AJ, Campisi G, Chaudhury P, Giannias B, et al. Hypertonic saline resuscitation of hemorrhagic shock diminishes neutrophil rolling and adherence to endothelium and reduces in vivo vascular leakage. Ann Surg. 2002;236(5):634-42. [
MedLine]
18. Lindeman JH, Abdul-Hussien H, Schaapherder AF, Van Bockel JH, Von der Thüsen JH, Roelen DL, et al. Enhanced expression and activation of pro-inflammatory transcription factors distinguish aneurysmal from atherosclerotic aorta: IL-6- and IL-8-dominated inflammatory responses prevail in the human aneurysm. Clin Sci (Lond). 2008;114(11):687-97. [
MedLine]
19. Middleton RK, Lloyd GM, Bown MJ, Cooper NJ, London NJ, Sayers RD. The pro-inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: a protein array study. J Vasc Surg. 2007;45(3):574-80. [
MedLine]
20. Gharavi NM, Alva JA, Mouillesseaux KP, Lai C, Yeh M, Yeung W, et al. Role of the Jak/STAT pathway in the regulation of interleukin-8 transcription by oxidized phospholipids in vitro and in atherosclerosis in vivo. J Biol Chem. 2007;282(43):31460-8. [
MedLine]
21. Lindholt JS, Shi GP. Chronic inflammation, immune response, and infection in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2006;31(5):453-63. [
MedLine]



 All scientific articles published at bjcvs.org are licensed under a Creative Commons license
All scientific articles published at bjcvs.org are licensed under a Creative Commons license