INTRODUCTION
Heart failure (HF) has been one of the greatest clinical challenges in the field of public health today, and is considered to be an epidemic in progress: it is diagnosed in 1% to 2% of the population in developed countries [1].
Statistics in the U.S. shows that heart failure affects about five million people, and it is estimated that about 400,000 new cases are diagnosed annually, representing 15 million hospitalizations at a cost of 10,000 dollars per hospitalization and 200,000 deaths per year [2].
In 1994, Bocchi [3] demonstrated the high mortality of patients with advanced heart failure when only drug therapy is used. This author reported in a three-year follow-up that mortality was 20%, 40%, 55% and 60% at six months, one year, two years and three years, respectively.
The natural history of heart failure has been responsible for severe prognosis and low quality of life in these severely ill patients [3]. The search for alternative methods or complements to drug therapy (which can alter the course of the disease) is a major challenge for researchers [4].
Heart transplantation has been the main surgical treatment offered to patients with advanced heart failure accompanied by severe functional and hemodynamic repercussion, resulting in a significant change in the prognosis of this disease [4,5].
Several factors prevent heart transplantation from being extended to a larger contingent of patients, such as the limited number of donors, the adverse effects of immunosuppression, the clinical and psychosocial conditions of the recipient, and other factors that can result in a patient being denied a transplant [5].
Thus, the search for other methods of surgical treatment remains constant on the part of researchers who are dedicated to treating this serious disease [6-13]. A new possibility then arose: surgically approaching the mitral valve in patients with severe left ventricular dysfunction in order to improve ventricular performance. This possibility may be included in the surgical and therapeutic tools to combat advanced heart failure [14-23].
The presence of mitral valve insufficiency in heart failure represents a predictor factor of mortality and a worsening in the patient's quality of life [24,25].
Some authors [14-23] have shown that the correction of mitral valve insufficiency in patients with severe left ventricular dysfunction - whether due to valvuloplasty or to valve replacement with preservation of subvalvar apparatus - was associated with low operative mortality and improves survival in the short- and mid-term.
In this study, we consider the data in the literature showing that the techniques of preserving the subvalvar apparatus in mitral valve replacement during heart failure may lead to an increase of ventricular function, improvement in functional class and short- and mid-term survival. We also consider studies that prove that the crossing of the papillary muscles and prosthesis implantation in mitral valve replacement, as proposed by Gomes [17], Gomes et al. [19], and Santana Filho [23]. The aim of this study is to analyze the short- and mid-term results for patients with mitral valve insufficiency who underwent mitral valve replacement with the technique of crossed papillopexy and annular constriction.
METHODS
With the approval by the Ethics Committee of the Federal University of Mato Grosso do Sul, 13 patients with heart failure in III or IV functional class (FC), according to the Criteria Committee of the New York Heart Association underwent mitral valve replacement with annular constriction and preservation of the subvalvar apparatus with the crossed papillopexy technique.
Patients were between 34 and 73 years of age, averaging 54.1 ± 10.8 years; four (31%) females and nine (69%) males. All patients were undergoing drug therapy (with the maximum acceptable dose of drugs). Etiologically, all patients presented idiopathic dilated cardiomyopathy with moderate or severe mitral insufficiency, ventricular dysfunction (Ejection Fraction <60% - Teicholz method) and III and IV Functional Class, as shown in Table 1. The exclusion criteria were associated ischemic heart disease, double valve replacement, or tricuspid valvuloplasty and emergency surgery.

Doppler echocardiography evaluation was performed in the preoperative period, in the first, third, sixth, and twelfth months, and after at every six months of postoperative. We analyzed the following parameters: Ejection Fraction, diastolic diameter of the left atrium, left ventricular end-diastolic and end-systolic diameter, left ventricular end-diastolic and end-systolic volume, percentage of left ventricular systolic shortening, left ventricular mass and volume-mass relationship in the left ventricle.
After anesthesia, the patients uderwent surgery using longitudinal median sternotomy, conventional cardiopulmonary bypass, moderate hypothermia (27ºC) and St. Thomas antegrade crystalloid cardioplegia at 4ºC. Next, left atriotomy (parallel to the interatrial septum) and inspection of the valve and subvalvar apparatus (Figure 1A) were performed. After, a circular incision at the base of the anterior cuspid (aiming to withdraw it from the mitral annulus and maintaining a 2mm cusp), and a subsequent central longitudinal incision of the anterior cuspid were performed (Figure 1B). Then, repair sutures (braided polyester coated with polybutilate 00 padded Teflon) were used in each half of the anterior cuspid (Figure 1C). Using this suture repair, the crossing of the papillary muscles through the attachment of opposite commissures - papillopexy cross (Figure 1D) - was performed.
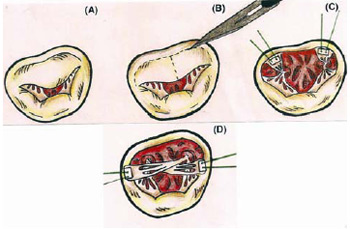
Fig. 1 - Crossed papillopexy - A. Native valve. B. Withdrawing of the anterior cuspid. C. Sutures on the half of anterior cuspid. D. Crossed papillopexy
Next, the measurement of the mitral annulus was performed, and surgeons agreed to choose an undersized prosthesis - one number below the measurement obtained - in order to complete annular constriction. Surgeons used enough braided polyester sutures coated with 00 polybutilate on the mitral annulus to implant the valvular prosthesis, attaching the cuspid after the ring itself in order to preserve the subvalvar apparatus. A left atrial suture was performed (using a one-layer continuous running suture) with polypropylene 000 (Figure 2).

Fig. 2 - Schematic illustration of the crossed papillopexy
In the postoperative period, the time of observation and follow-up ranged from one to 30 months, based on the functional class of heart failure (NYHA), the actuarial survival curve, and Doppler echocardiography (performed in the first, third and sixth months and every six months during the postoperative period). The statistical method used was the Wilcoxon, with a level of significance of 0.05.
RESULTS
There were no deaths during surgery or in the immediate postoperative period. In the long-term follow-up, 11 (84.6%) patients were discharged from hospital with significant evidence of clinical and echocardiographic improvement compared to the preoperative condition. Two (15.4%) patients died in the second month of postoperative due to bronchopneumonia, followed by septic shock and multiple organ failure.
Four (30.8%) bovine pericardium bioprostheses (Braile Biomédica) were implanted, and in nine (69.2%) patients, mechanical prostheses (St. Jude Medical) were used. Smaller-sized prostheses were chosen in an attempt to remodel the base of the left ventricle. The cardiopulmonary bypass time ranged from 49.3 ± 6.51 minutes. There was no need to use intra-aortic balloon.
Doppler echocardiographic parameters, according to Table 2 and Figure 3, show improvement in ventricle geometry and atrial and left ventricular function, as compared to the preoperative period.

Fig. 3 - Postoperative echocardiographic image showing the crossing of the papillary muscles
All patients presented significant clinical improvement. According to the NYHA classification, in the postoperative period, ten (76.9%) patients were in IV Functional Class and three (23.1%) in III Functional Class. In the postoperative evaluation, five (45.5%) patients were in II Functional Class and six (54.5%), in I Functional Class.
Survival was evaluated by actuarial curve, considering patients alive at 1, 6, 12, 24 and 30 months of follow-up after surgery, respectively represented by 100%, 82.6%, 71.6%, 71.6% and 71.6%, as shown in Figure 4.
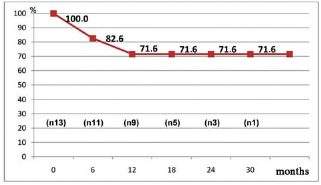
Fig. 4 - Actuarial survival curve of patients who underwent crossed papillopexy and mitral valve replacement with annular constriction with severe heart failure. n=number
In this study, we emphasize the correction of mitral valve insufficiency with the crossed papillopexy technique in patients with ventricular dysfunction, and preservation of the subvalvar apparatus and implantation of an undersized valve prosthesis to remodel the base of the left ventricle and the subvalvar apparatus aiming to improve ventricular performance.
In this study, using the cross papillopexy technique and mitral valve replacement with annular constriction in patients with severe heart failure with moderate-to-severe mitral valve insufficiency, there was no operative mortality, and no need for mechanical ventricular assistance in the postoperative; the survival was 82.6% at six months, 71.6% in one year and 71.6% in 30 months; there was substantial increase of the Ejection Fraction of 34% to 45%; and there was a decrease in ventricular diameters and improvement of Functional Class.
The absence of operative mortality and mechanical ventricular assistance mechanism presented in this study, compared with the other papers previously mentioned [14-23], may be explained by the fact that the crossing of the papillary muscles has allowed for left ventricular diastolic containment. Similarly, the annular constriction due to the implantation of an undersized prosthesis promotes the base ventricular remodeling, allowing improvement of cardiac performance.
It is possible that the increase the Ejection Fraction using the crossed papillopexy technique and mitral valve replacement with annular constriction in patients with heart failure with moderate-to-severe mitral valve insufficiency has been greater than in the studies previously mentioned because of the difference in the geometry of the left ventricular cavity using diastolic containment after crossing the papillary muscles. With this technique, there is a decrease in the angle of displacement of the bases of the papillary muscles, providing a geometric support by reducing pendular displacement, thus avoiding the passive ventricular dilation.
The higher survival rates shown in this study compared with other mitral valve approaches forementioned [14-23] is probably due to the immediate improvement of ventricular performance in short-term (this factor may be considered to be a predictor of increased survival). It is possible that in a long-term follow-up, this improvement of ventricular performance can be a determining factor in increased survival rates.
Regarding the improvement in Functional Class, it may be observed that the results found in the literature are similar to this study, whereas most of the techniques proposed are able to correct the mitral valve insufficiency - a predominant symptom in these patients.
In the overall analysis, respecting the limitations of this study (which was a non-randomized study, no comparative, with a small sample and with short- and mid-term follow-up), this technique presented herein proved to be feasible and reproducible, showing significant improvement in Functional Class and in echocardiographic parameters. Moreover, the technique used in this study provides satisfactory survival, showing evidence of favorable cardiac remodeling and significant functional left ventricle recovery in patients with heart failure and patients with moderate-to-severe mitral valve insufficiency.
REFERENCES
1. Tendera M. Epidemiology, treatment, and guidelines for the treatment of heart failure in Europe. Eur Heart J. 2005;7(Suppl. J):J5-J9.
2. Bundkirchen A, Schwinger RHG. Epidemiology and economic burden of chronic heart failure. Eur Heart J. 2004;6(Suppl. D):D57-D60.
3. Bocchi EA. Situação atual das indicações e resultados do tratamento cirúrgico da insuficiência cardíaca. Arq Bras Cardiol. 1994;63(6):523-30. [
MedLine]
4. Stolf NAG, Jatene AD. História do transplante cardíaco. Rev Soc Cardiol Estado de São Paulo. 1995;5:609-13.
5. Taylor DO, Edwards LB, Boucek MM, Trulock EP, Deng MC, Keck BM, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-second official adult heart transplant report-2005. J Heart Lung Transplant. 2005;24(8):945-55. [
MedLine]
6. Moreira LF, Stolf NA, Braile DM, Jatene AD. Dynamic cardiomyoplasty in South America. Ann Thorac Surg. 1996;61(1):408-12. [
MedLine]
7. Braile DM, Godoy MF, Thèvenard GH, Thèvenard RH, Braile MC, Leal JC, et al. Dynamic cardiomyoplasty: long-term clinical results in patients with dilated cardiomyopathy. Ann Thorac Surg. 2000;69(5):1445-7. [
MedLine]
8. Carpentier A, Chachques JC. Clinical dynamic cardiomyoplasty: method and outcome. Semin Thorac Cardiovasc Surg. 1991;3(2):136-9. [
MedLine]
9. Batista RJ, Verde J, Nery P, Bocchino L, Takeshita N, Bhayana JN, et al. Partial left ventriculectomy to treat end-stage heart disease. Ann Thorac Surg. 1997;64(3):634-8. [
MedLine]
10. Pontes JCDV, Gomes OM, Medeiros CGS, Silva AF, Duarte JJ, Gardenal N, et al. Ventriculectomia parcial esquerda: operação de Batista em pacientes acima de 60 anos. Rev Bras Cir Cardiovasc. 2001;16(1):20-7.
11. Bax JJ, Abraham T, Barold SS, Breithardt OA, Fung JW, Garrigue S, et al. Cardiac resynchronization therapy: Part 1issues before device implantation. J Am Coll Cardiol. 2005;46(12):2153-67. [
MedLine]
12. Brofman PR, Carvalho KA, Guarita-Souza LC, Rebelatto C, Hansen P, Senegaglia AC, et al. Transplante celular: análise funcional, imunocitoquímica e histopatológica em modelo experimental de miocardiopatia isquêmica utilizando diferentes células. Rev Bras Cir Cardiovasc. 2004;19(3):261-6.
13. DeBakey ME. Development of mechanical heart devices. Ann Thorac Surg. 2005;79(6):S2228-31. [
MedLine]
14. Bolling SF, Deeb GM, Brunsting LA, Bach DS. Early outcome of mitral valve reconstruction in patients with end-stage cardiomyopathy. J Thorac Cardiovasc Surg. 1995;109(4):676-82.
15. Bolling SF, Pagani FD, Deeb GM, Bach DS. Intermediate-term outcome of mitral reconstruction in cardiomyopathy. J Thorac Cardiovasc Surg. 1998;115(2):381-6.
16. Calafiore AM, Gallina S, Contini M, Iacò A, Barsotti A, Gaeta F, et al. Surgical treatment of dilated cardiomyopathy with conventional techniques. Eur J Cardiothorac Surg. 1999;16(Suppl 1):S73-8. [
MedLine]
17. Gomes OM. Papilopexia para prevenção da degeneração miocárdica após substituição mitral. Arq Bras Cardiol. 1987;49(suppl 1):165. [
MedLine]
18. Buffolo E, Paula IA, Palma H, Branco JN. A new surgical approach for treating dilated cardiomyopathy with mitral regurgitation. Arq Bras Cardiol. 2000;74(2):129-40. [
MedLine]
19. Gomes OM, Gomes ES, Santana Filho GP, Pontes JCDV, Benfatti RA. Nova abordagem técnica para papilopexia cruzada em operação de substituição valvar mitral: resultados imediatos. Rev Bras Cir Cardiovasc. 2005;20(3):340-5.
20. Breda JR, Palma JHA, Teles CA, Branco JNR, Catani R, Buffolo E. Miocardiopatia terminal com insuficiência mitral secundária: tratamento com implante de prótese e remodelamento interno do ventrículo esquerdo. Rev Bras Cir Cardiovasc. 2006;21(3):283-8.
21. Puig LB, Gaiotto FA, Oliveira Jr. JL, Pardi MM, Bacal F, Mady C, et al. Mitral valve replacement and remodeling of the left ventricle in dilated cardiomyopathy with mitral regurgitation: initial results. Arq Bras Cardiol. 2002;78(2):224-9. [
MedLine]
22. Gaiotto FA, Puig LB, Mady C, Fernandes F, Tossuniam CE, Pardi MM, et al. Substituição da valva mitral com tração dos músculos papilares em pacientes com miocardiopatia dilatada. Rev Bras Cir Cardiovasc. 2007;22(1):68-74. [
MedLine]
23. Santana Filho GP. Influência da técnica de papilopexia cruzada nas funções atrial e ventricular esquerdas após substituição da valva mitral: estudo ecodopplecardiográfico [Tese de Mestrado]. Belo Horizonte:Fundação Cardiovascular São Francisco de Assis;2006.
24. Blondheim DS, Jacobs LE, Kotler MN, Costacurta GA, Parry WR. Dilated cardiomyopathy with mitral regurgitation: decreased survival despite a low frequency of left ventricular thrombus. Am Heart J. 1991;122(3 Pt [part]1[/part]):763-71. [
MedLine]
25. Cioffi G, Tarantini L, De Feo S, Pulignano G, Del Sindaco D, Stefenelli C, et al. Functional mitral regurgitation predicts 1year mortality in elderly patients with systolic chronic heart failure. Eur J Heart Fail. 2005;7(7):1112-7. [
MedLine]






 All scientific articles published at bjcvs.org are licensed under a Creative Commons license
All scientific articles published at bjcvs.org are licensed under a Creative Commons license