INTRODUCTION
Aortic coarctation is defined as the narrowing of the descending aorta just below the origin of the left subclavian artery in a region called the isthmus, where the ductus arteriosus is connected by proximity. The disease may develop alone, or it may be associated with intracardiac anomalies or aortic arch hypoplasia.
The surgical basics of the first correction of aortic coarctation in newborns were presented by Mustard et al. in 1953 [1]. Since then, we have seen a gradual improvement in outcomes. However, the correction of coarctation with aortic arch hypoplasia still represents a challenge for surgeons [2].
The definition of aortic arch hypoplasia was proposed by Moulaert et al. [3]. The aortic arch is considered hypoplastic when its diameter reaches 50% of the diameter of the ascending aorta. Hypoplasia can be distal (isthmian - type A) or proximal/distal (transverse arch - Type B - Figure 1). When the aortic coarctation is associated with the arch hypoplasia, we can conclude that it deals with duct-dependent heart disease, where, during fetal development, there was less infusion in this region caused by left-right blood flow, which resulted in hypodevelopment of the arc and the consequent migration of ductal cells in the isthmian region [3].

Fig, 1 - Preductal aortic coarctation with hypoplasia of the aortic segment between the left carotid arteries and left subclavian
Current studies show that surgeons typically agree on the total expansion of the aortic arch, but there is no consensus on which surgical technique to use for this expansion [2,4,5].
Recent progress in neonatal intensive care and in two-dimensional Echo-Doppler techniques have allowed for the correction of complex intracardiac malformations associated with aortic arch abnormalities in a single stage, and with satisfactory results [6-8]. In these cases, aortic coarctation is surgically approached as an interruption of the aortic arch, whether associated with arch hypoplasia or not, and surgeons use cardiopulmonary bypass with deep hypothermia and circulatory arrest.
Other techniques have been described for correction of this malformation [4,9,10], but significant residual gradients were observed, frequent recoarctations and growth deficit in the left superior limb, significantly when the procedure of occlusion or patch of the subclavian artery is used [4, 11]. In addition to this is the common denominator of these techniques: the preservation of the hypoplastic zone, in which growth is restricted in long-term [12], which may cause retraction.
The aim of this study is to report a new technique for correction of coarctation with aortic arch hypoplasia type B described by Moulaert. This technique preserves the vessels of base, resects the hypoplastic zone while providing a good hemodynamic profile. Cases of isolated isthmian coarctation or with hypoplasia type A were not included in this study because these pathological entities can be treated with conventional techniques of resection and end-to-end anastomosis with good outcomes. In our view, the real challenge for the surgeon is type B, which is a more complex type of hypoplasia.
METHODS
Patients
Between January 2005 and July 2006, nine newborns with aortic coarctation and aortic arch hypoplasia type B (Moulaert) were admitted to the ICU (during use of prostaglandin in continuous infusion aiming to maintain the permeability of the ductus arteriosus, developing into presentation of heart failure, arterial hypertension and metabolic acidosis). They underwent surgical correction with the technique to be described. Three patients presented small interventricular communication. The patients'ages ranged from 3 to 20 days, with a mean age of 7 days. All patients underwent transthoracic echocardiogram prior to the operation. No hemodynamic studies were performed.
Technique
The surgical approach for all patients was the left posterolateral thoracotomy. The left lung is pulled away, and the descending aorta, ductus arteriosus and vessels of the base are widely dissected up to the ascending aorta (Figure 2). The ductus arteriosus and the first two or three intercostal branches are connected with 6-0 polypropylene for wide mobility of the descending aorta (Figures 3 and 4). A 0.5 mg/kg dose of heparin is administered. Aortic clamping is performed immediately after the brachiocephalic trunk and in descending aorta, after emergence of the second or third intercostal branch (previously connected). The left carotid arteries and subclavian are clamped. The subclavian artery is then sectioned at the base of its implantation on the aorta (Figure 5). The distal segment remains clamped. The coarctation area and, specifically, the hypoplastic segment, located between the subclavian artery and the left carotid artery, are widely resected (Figure 6). A small opening in the concavity of the arc toward the ascending aorta is performed in order to enlarge the maximum area of anastomosis. Subsequently, an end-to-end anastomosis is performed between the aortic arch and the descending aorta with continuous 7-0 PDS suture (Figure 7).

Fig.2 - Surgical anatomy of aortic coarctation with arch hypoplasia

Fig. 3 - Intercostal arteries occlusion

Fig.4 - Ductus arteriosus occlusion
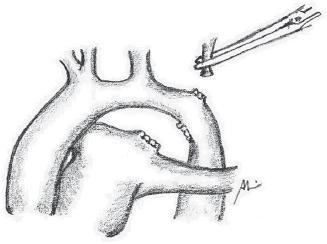
Fig. 5 - Section of the left subclavian artery

Fig. 6 - Wide resection of the coarctation area and the hypoplastic aortic arch
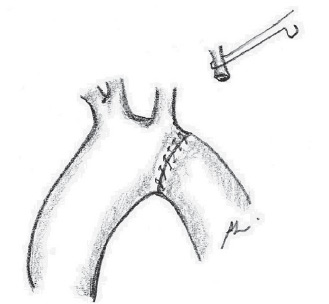
Fig. 7 - End-to-end anastomosis between the crest and the decending aorta
The forceps are removed and the anastomosis is revised. Partial clamping of the left carotid artery is then performed in its medium third, where surgeons perform a vertical incision in its wall. The subclavian artery is reimplanted in the carotid artery through lateroterminal anastomosis with continuous 7-0 PDS suture (Figure 8).

Fig. 8 - Lateroterminal reimplantation of the subclavian artery in the left carotid artery
There were no surgical mortalities, medullary ischemic accidents or neurological sequels caused by the partial clamping of the left carotid artery. The aortic clamping time was 25
+ 11 minutes.
The postoperative hospital stay ranged from 7 to 19 days, with mean of 11.7
+ 1.1 days.
Control Doppler echocardiogram performed just before discharge and at 3 and 6 months after the operation showed no gradient in six patients (Figure 9) and residual gradient (10mmHg) in three.
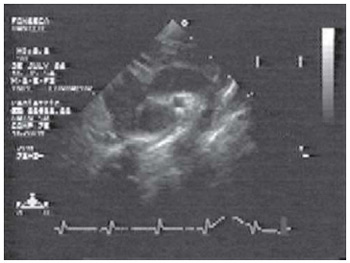
Fig. 9 - Control Echo-Doppler in operated patient, showing aortic arch after surgical correction without residual stenosis
All patients received clinical follow-ups, and at the time of this article, there have been no deaths or late complications.
DISCUSSION
The incidence of the aortic arch hypoplasia in coarctation ranges from 65% to 81% [13]. The introduction of therapy with prostaglandins in the neonatal period changed the history of this anomaly [9] with significant improvement in the results. Anatomopathological [14] and surgical [2,4,15] studies reported the clear obstruction created by aortic arch hypoplasia.
A high percentage of recoarctation in newborns was reported after correction with end-to-end anastomosis [16],
flapping of the subclavian artery [11] and a patch made of synthetic tissue [17]. Even the technique of extending the end-to-end anastomosis [18-20] requires recoarctation in more than 10% of cases.
Histological studies confirm the abnormal structure of the hypoplastic aortic arch. It is characterized by high percentage of collagen fibers in relation to the aorta's diameter and the limited presence of alpha-actin positive cells, which are responsible for vessel growth [12]. This reinforces the theory that, to achieve a satisfactory result, the surgical technique should include total resection of the hypoplastic area and ductus arteriosus.
Other techniques have been described [9,10] with apparently good immediate results. However, both techniques preserve the structure of the hypoplastic aortic arch, which may result in limited growth or retraction of the area around the anastomosis, causing recoarctation [12], or which may permanently sacrifice the left subclavian artery with the consequent growth deficit in the superior left limb and the consequences that this may cause [13]. Other authors prefer to treat this anomaly as an interruption of the aortic arch, using sternotomy, cardiopulmonary bypass and deep hypothermia [8]. In our view, this procedure is not necessary, except in the case of associated intracardiac abnormality, which may require single stage correction. After approaching the coarctation with aortic arch hypoplasia in all its aspects, we propose a technique without use of cardiopulmonary bypass. This technique takes into account the factors that are directly responsible for the high occurrence of recoarctations. This technique is based on the following principles: 1) it completely removes the ductal tissue; 2) it ressects the hypoplastic area of the arch and its pathological tissues with low growth potential; 3) it preserves the vessels at the basis through the left subclavian artery reimplantation; and 4) it does not generate gradient during the anastomosis.
CONCLUSION
From a technical point of view, we believe that the surgery proposed herein is feasible if there is good mobilization of the descending aorta. This can be achieved through wide dissection and occlusion of the first intercostal branches. Otherwise, it seems difficult to lead the distal segment toward the implantation base of the left carotid artery and to perform a tension-free end-to-end anastomosis. In this series, we did not have accidents or ischemic medullary sequels; however, this is always a possibility, and additional clinical observations are still needed.
Despite the small number of cases and short follow-up, the technique without use of cardiopulmonary bypass and without use of synthetic patch or prosthesis can be an excellent option for the treatment of this complex group of patients and can contribute to the improvement of immediate and mid-term clinical outcomes.
REFERENCES
1. Diethrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg. 1996;3(1):42-62. [
MedLine]
2. Roubin GS, New G, Iyer SS, Vitek JJ, Al-Mubarak N, Liu MW, et al. Immediate and late clinical outcomes of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery stenosis: a 5-year prospective analysis. Circulation. 2001;103(4):532-7. [
MedLine]
3. Hammer FD, Lacroix V, Duprez T, Grandin C, Verhelst R, Peeters A, et al. Cerebral microembolization after protected carotid artery stenting in surgical high-risk patients: results of a 2-year prospective study. J Vasc Surg. 2005;42(5):847-53.
4. Rapp JH, Wakil L, Sawhney R, Pan XM, Yenari MA, Glastonbury C, et al. Subclinical embolization after carotid artery stenting: new lesions on diffusion-weighted magnetic resonance imaging occur postprocedure. J Vasc Surg. 2007;45(5):867-72.
5. Piñero P, González A, Mayol A, Martínez E, González-Marcos JR, Boza F, et al. Silent ischemia after neuroprotected percutaneous carotid stenting: a diffusion-weighted MRI study. AJNR Am J Neuroradiol. 2006;27(6):1338-45. [
MedLine]
6. Faraglia V, Palombo G, Stella N, Taurino M, Iocca ML, Romano A, et al. Cerebral embolization in patients undergoing protected carotid-artery stenting and carotid surgey. J Cardiovasc Surg (Torino). 2007;48(6):683-8. [
MedLine]
7. Poppert H, Wolf O, Resch M, Theiss W, Schmidt-Thieme T, Graefin von Einsiedel H, et al. Differences in number, size and location of intracranial microembolic lesions after surgical versus endovascular treatment without protection device of carotid artery stenosis. J Neurol. 2004;251(10):1198-203. [
MedLine]
8. Schlüter M, Tübler T, Steffens JC, Mathey DG, Schofer J. Focal ischemia of the brain after neuroprotected carotid artery stenting. J Am Coll Cardiol. 2003;17:42(6):1007-13.
9. Mathur A, Roubin GS, Gomez CR, Iyer SS, Wong PM, Piamsomboon C, et al. Elective carotid artery stenting in the presence of contralateral occlusion. Am J Cardiol. 1998;81(11):1315-7. [
MedLine]
10. Perler BA, Dardik A, Burleyson GP, Gordon TA, Williams GM. Influence of age and hospital volume on the results of carotid endarterectomy: a statewide analysis of 9918 cases. J Vasc Surg. 1998;27(1):25-31. [
MedLine]
11. Hobson RW 2nd, Howard VJ, Roubin GS, Brott TG, Ferguson RD, Popma JJ, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004;(40):1106-11.
12. Sundt TM Jr, Ebersoldt MJ, Sharbrough FW, Piepgras DG, Marsh WR, Messick JM Jr, et al. The risk-benefit ratio of intraoperative shunting during carotid endarterectomy. Relevancy to operative and postoperative results and complications. Ann Surg. 1986;203(2):196-204. [
MedLine]
13. Riles TS. Surgical management of internal carotid artery stenosis: preventing complications. Can J Surg. 1994;37(2):124-7. [
MedLine]
14. Hertzer NR, O'Hara PJ, Mascha EJ, Krajewski LP, Sullivan TM, Beven EG. Early outcome assessment for 2228 consecutive carotid endarterectomy procedures: the Cleveland Clinic experience from 1989 to 1995. J Vasc Surg. 1997;26(1):1-10. [
MedLine]
15. CAVATAS investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357(9270):1729-37. [
MedLine]
16. Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351(15):1493-501. [
MedLine]
17. SPACE Collaborative Group, Ringleb PA, Allenberg J, Brückmann H, Eckstein HH, Fraedrich G, Hartmann M, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368(9543):1239-47. [
MedLine]
18. Mozes G, Sullivan TM, Torres-Russotto DR, Bower TC, Hoskin TL, Sampaio SM, et al. Carotid endarterectomy in SAPPHIRE-eligible high-risk patients: implications for selecting patients for carotid angioplasty and stenting. J Vasc Surg. 2004;39(5):958-65.
19. Gasparis AP, Ricotta L, Cuadra SA, Char DJ, Purtill WA, Van Bemmelen PS, et al. High-risk carotid endarterectomy: fact or fiction. J Vasc Surg. 2003;37(1):40-6. [
MedLine]
20. Diethrich EB. Carotid endarterectomy is better than carotid stenting for asymptomatic patients. Pro position. Tex Heart Inst J. 2006;33(2):209-10. [
MedLine]
21. Diethrich EB. What happened to carotid stenting and will it be permanent? Anais Syllabus,CICE;2008.
22. Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355(16):1660-71. [
MedLine]
23. Perler BA, Willians GM. Carotid endarterectomy in the very elderly: is it worthwhile? Surgery. 1994;116(3):479-83. [
MedLine]
24. O'Hara PJ, Hertzer NR, Mascha EJ, Beven EG, Krajewski LP, Sullivan TM. Carotid endarterectomy in octogenarians: early results and late outcome. J Vasc Surg. 1998;27(5):860-9.
25. Rockman CB, Jacobowitz GR, Adelman MA, Lamparello PJ, Cagne PJ, Landis R, et al. The benefits of carotid endarterectomy in the octogenarian: a challenge to the resuts of carotid angioplasty and stenting. Ann Vasc Surg. 2003;17(1):9-14. [
MedLine]
26. Bonamigo TP, Lucas ML. Análise crítica das indicações e resultados do tratamento cirúrgico da doença carotídea. J Vasc Bras. 2007;6(4):366-77.
27. European Carotid Surgery Trialists' Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70-99%) or with mild (029%) carotid stenosis. Lancet. 1991;337(8752):1235-43. [
MedLine]
28. Bonamigo TP, Weber EL, Lucas ML, Bianco C, Cardozo MA. Carotid endarterectomy in patients with contralateral occlusion: a 10-year experience. J Vasc Bras. 2004;3(1):83-91.
29. Samson RH, Showalter DP, Yunis JP. Routine carotid endarterectomy without a shunt, even in the presence of a contralateral occlusion. Cardiovasc Surg. 1998;6(5):475-84. [
MedLine]









 All scientific articles published at bjcvs.org are licensed under a Creative Commons license
All scientific articles published at bjcvs.org are licensed under a Creative Commons license