Application of ultrasound energy by an endarterectomy probe can facilitate the removal of atheromatous plaque, but the effect of this procedure on surrounding vessel structure and function is still a matter of experimental investigations.
To determine whether ultrasound energy impairs the production of nitric oxide or damages vascular smooth muscle function, isolated canine epicardial coronary artery segments were exposed to either high (25 W) or low (0-10 W) ultrasonic energy outputs, for 15 seconds, using an endarterectomy device prototype. After exposure, segments of epicardial coronary artery were studied in organ chambers. The following drugs were used: adenosine diphosphate (ADP), acetylcholine (Ach) and sodium fluoride (NaF) to study endothelium-dependent relaxation and sodium nitroprusside (SNP) and isoproterenol to evaluate endothelium-independent relaxation.
Application of high ultrasonic energy power impaired endothelium-dependent relaxation to ADP (10
M) and NaF (0.5 - 9.5 mM) in epicardial coronary arteries. However, low ultrasound energy output at the tip of the probe did not alter the endothelium-dependent relaxation (either maximal relaxation or EC
) to the same agonists. Vascular smooth muscle relaxation to isoproterenol (10
M) was unaltered following exposure to either low or high ultrasonic energy outputs.
These experiments currently prove that ultrasonic energy changes endothelial function of epicardial coronary arteries at high power. However, ultrasound does not alter the ability of vascular smooth muscle of canine epicardial coronary arteries to relax.
Aplicação de energia por ultra-som pode facilitar a remoção da placa ateromatosa, mas o efeito desse procedimento em vasos próximos ainda é matéria de estudos experimentais.
Para determinar se a energia ultra-sônica compromete a produção de óxido nítrico, segmentos de artérias coronárias caninas foram expostos a baixos (0-10 W) e altos (25 W) níveis de energia por 15 segundos, utilizando-se protótipo de aparelho para a realização de endarterectomia. Após exposição, segmentos das artérias coronarianas foram estudados em organ chambers. Para os ensaios farmacológicos foram utilizadas as seguintes drogas: difosfato de adenosina (ADP), acetilcolina (Ach) e fluoreto de sódio (NaF) para a avaliação do relaxamento dependente do endotélio. O nitroprussiato de sódio (NPS) e o isoproterenol foram utilizados para a avaliação do relaxamento independente do endotélio.
A aplicação de alta energia ultra-sônica comprometeu o relaxamento dependente do endotélio induzido por ADP (10
M) e NaF (0,5 - 9,5 mM) em artérias coronarianas epicárdicas. Entretanto, baixos valores de energia ultra-sônica não alteraram o relaxamento dependente do endotélio (nem o relaxamento máximo e nem a EC
) induzido pelos mesmos agonistas. O relaxamento da musculatura lisa vascular induzido por isoproterenol (10
M) não foi comprometido, tanto por baixos, quanto por altos níveis de energia ultra-sônica.
Os experimentos demonstram que altas energias ultra-sônicas alteram a função endotelial. Entretanto, o ultra-som não altera a habilidade de relaxamento da musculatura lisa vascular de artérias caninas epicárdicas.
INTRODUCTION
Diffuse atherosclerotic coronary artery disease may not be amenable to standard methods of arterial grafting, and coronary artery endarterectomy may be necessary to remove atheromatous plaques that compromise arterial runoff [1,2]. Endarterectomy is a technically demanding procedure in which the surgeon removes the core of plaque to improve the luminal size and quality of the recipient vessel. Numerous methods have been used to facilitate endarterectomy including specially designed probes, carbon dioxide insufflation which dissects the plaque from the remained. Endareterectomy also entails the risks of increased morbidity and mortality [2,3].
Application of intraarterial ultrasound has been shown to be feasible for use in recanalization of calcified atherosclerotic occlusions in vitro, in animals and in humans [4,5]. The use of ultrasonic waves as an adjunct to manual endarterectomy can improve the effectiveness of the procedure by facilitating the removal of the atheromatous plaque completely from the arterial wall. However, very little is known about possible damaging effects of ultrasound energy application on epicardial coronary artery function.
The intima of the coronary artery produces an endothelium-derived relaxing factor (EDRF) [6]. The EDRF was identified as nitric oxide (NO) [7], which acts as an endogenous coronary nitrovasodilator [8,9], in addition to inhibiting platelet adhesion [10,11] and aggregation [12,13] in the blood vessel. Damaging effect of ultrasonic waves on the function of the coronary artery endothelium and vascular smooth muscle could impair the production of NO and increase the risk of coronary artery vasospasm and thrombosis. In the present investigation, we evaluated the effect of ultrasound energy on production of NO and vascular smooth muscle function of the epicardial coronary artery in vitro.
METHODS
Animal Preparation
Six heartworm-free mongrel dogs (n=6) (25-30 kg) of either sex were anesthetized with pentobarbital sodium (30 mg/kg intravenous injection; Fort Dodge Laboratories, Inc., Fort Dodge, Iowa), and exsanguinated via carotid arteries. The chest was opened, and the beating heart was harvested quickly and immersed in cool, oxygenated physiological salt solution of the following composition (mM): NaCl 118.3, KCl 4.7, MgSO
4 1.2, KH
2PO
4 1.22, CaCl
2 2.5, NaHCO
3 25.0, and glucose 11.1. The procedures and handling of the animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Mayo Foundation.
In Vitro Experiments
Vascular segments preparation:
The left circumflex coronary artery was carefully dissected free and placed in control solution. The artery was sectioned into eight rings (4 - 5 mm in length) with special care taken not to touch the intimal surface of the vascular segments. In four of these segments, in which vascular smooth muscle function was to be tested without the influence of the endothelium, the endothelium was removed by gently rubbing the intimal surface of the blood vessel with a pair of watchmakers' forceps. This procedure removes endothelium but does not affect ability of vascular smooth muscle to contract or relax [14,15]. In this manner, four pairs of coronary artery rings (with and without endothelium) from the same animal were studied in parallel in our eight-bath organ chamber system.
Ultrasonic Device and Experimental Groups
These experiments were performed using the KUE (King Ultrasonic Endarterectomy) device (Heart Tech of Minnesota, Minneapolis, MN) (Figure 1). The energy was transmitted to the vascular wall by a stainless steel endarterectomy probe at the time the probe was in contact with the intima of artery segments while they were immersed in physiological salt solution (Figure 2).

Fig. 1 - King Ultrasonic Endarterectomy (KUE) device
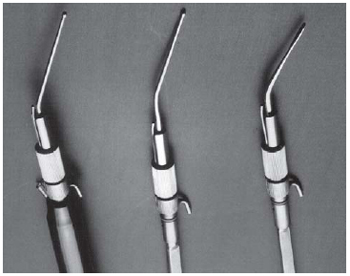
Fig. 2 - The endarterectomy probes of KUE device
Experiments were performed at probe-tip power outputs of 0-10 W or 25 W correspond to low and high energy levels of the device, respectively. The ultrasonic power that was actually delivered to the blood vessel wall was not directly measured.
After arterial rings were prepared and immersed in physiologic salt solution (as described earlier), they were exposed to ultrasonic energy by placing the tip of the probe into the lumen of the vessel. All the rings were treated one by one in the same manner, and then were suspended in organ chambers. The Lowest energy level of the device was used in one group, and highest energy was used in the other, in order to determine the effect of different energy outputs at the tip of the probe. Ultrasonic energy was delivered for 15 seconds. Two pairs of coronary artery segments were used in each group and one of these pairs was treated with the probe only by placing it into the lumen of the vessel without transmitting ultrasound energy, serving as a control of the influence of possible mechanical trauma of the probe itself.
Vascular reactivity study in organ chambers
Each ring was suspended by two stainless steel clips passed through its lumen. Vascular segments, with and without endothelium, were immersed in organ chambers (25 ml) filled with control solution maintained at 37°C and bubbled with 95% O
2 and 5% CO
2 (pH = 7.4). One clip was anchored to the bottom of the organ chamber, and the other was connected to a strain-gauge for measurement of isometric force (Statham UC 2, Gould, Cleveland, Ohio). The rings were placed at the optimal point of their length-tension relation by progressively stretching them until contraction to potassium ions (20 mM), at each level of distension, was maximal [13,14]. After optimal tension was achieved, the coronary artery rings were allowed to equilibrate in control solution for 30-45 minutes before administration of drugs. In all experiments, the presence or absence of endothelium was confirmed by determining the response to acetylcholine (10
-6 M) in rings contracted with potassium ions (20 mM) [6]. In all experiments, indomethacin (10
-6 M) was added to the organ chambers 40 minutes prior to the administration of drugs to prevent synthesis of endogenous prostanoids and to warrant that relaxant effects were due NO action, as NO release is not inhibited by blockers of cyclooxygenase [6].
Drugs
The following drugs were used: acetylcholine chloride (Ach), adenosine diphosphate (ADP), indomethacin, isoproterenol hydrochloride, sodium fluoride (NaF), prostaglandin F
2a (PGF
2a), and sodium nitroprusside (all from Sigma Chemical Company, St. Louis, Missouri). All drugs were prepared with distilled water except for indomethacin, which was dissolved in em Na
2CO
3 (10
-5 M). The concentrations are expressed as final molar concentration in the organ chambers.
Data analysis
Results are expressed as mean ± SEM. In all experiments, n refers to the number of animals from which blood vessels were taken. In segments contracted with PGF
2a, relaxation responses are expressed as percent changes from the contracted levels. Statistical evaluation of data was performed using the two-way ANOVA and Bonferroni post-test for multiple comparisons. Differences were considered to be statistically significant when p was less than 0.05.
RESULTS
Endothelium-dependent relaxation
Adenosine diphosphate
ADP (10
-9 - 10
-4 M) induced concentration-dependent relaxation in control and all experimental coronary artery segments with endothelium which had been contracted with PGF
2a. ADP caused a slight decrease in tension in coronary artery segments without endothelium. Exposure to low intensity ultrasound energy output (0-10 W) did not alter the maximal relaxation or sensitivity to ADP-mediated relaxation in coronary artery segments with or without endothelium. However, exposure to high intensity ultrasound energy output (25 W) impaired the maximal relaxation and sensitivity to ADP-mediated relaxations in coronary artery segments with endothelium (Figure 3, p < 0.05, n = 6 in each group).
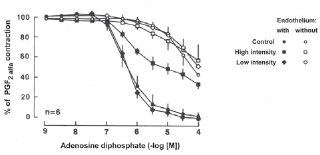
Fig. 3 - Endothelium-dependent relaxation to adenosine diphosphate (ADP) in the canine coronary artery. Canine coronary arteries were exposed to either low or high intensity ultrasonic energy and contracted with prostaglandin F
2a (PGF
2a). When the PGF
2a contraction was stable, arteries were exposed to increasing concentrations of ADP. Data are presented as means ± SEM, n = 6
Ach (10
-9 - 10
-4 M) induced comparable, concentration-dependent relaxation in control and all experimental coronary artery segments with endothelium which had been contracted with PGF
2a. Ach produced no significant change in tension in coronary artery segments without endothelium. Exposure to low intensity ultrasound energy output (0-10 W) did not alter the maximal relaxation or sensitivity to Ach-induced relaxation in coronary artery segments with endothelium. However, as with ADP, relaxation was impaired in coronary artery segments with endothelium exposed to high intensity ultrasound energy output (25 W) (Figure 4, p < 0.05, n = 6 in each group).
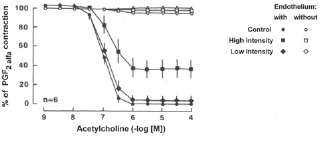
Fig. 4 - Endothelium-dependent relaxation to acetylcholine (Ach) in the canine coronary artery. Canine coronary arteries were exposed to either low or high intensity ultrasonic energy and contracted with prostaglandin F
2a (PGF
2a). When the PGF
2a contraction was stable, arteries were exposed to increasing concentrations of Ach. Data are presented as means ± SEM, n = 6
NaF (0.5 - 9.5 mM) induced concentration dependent relaxation in canine coronary arteries with endothelium which had been contracted with PGF
2a. However, exposure to NaF increased tension in coronary arteries without endothelium. In coronary artery segments with endothelium exposed to low intensity ultrasound energy (0-10 W), NaF-induced concentration-dependent relaxations were comparable to control arteries treated only mechanically. In contrast, in coronary artery segments with endothelium exposed to high intensity ultrasound energy output (25 W), NaF-induced concentration dependent relaxations were significantly less than relaxations in arteries exposed to low ultrasonic energy or control rings (Figure 5, p < 0.05, n = 6 in each group).

Fig. 5 - Endothelium-dependent relaxation to sodium fluoride (NaF) in the canine coronary artery. Canine coronary arteries were exposed to either low or high intensity ultrasonic energy and contracted with prostaglandin F
2a (PGF
2a). When the PGF
2a contraction was stable, arteries were exposed to increasing concentrations of NaF. Data are presented as means ± SEM, n = 6
Cyclic GMP-mediated
Sodium nitroprusside (10
-9 - 10
-6 M) induced comparable concentration-dependent relaxation in control and experimental coronary artery segments without endothelium. Either low or high intensity ultrasound energy output did not alter the maximal relaxation induced by sodium nitroprusside or change the sensitivity of the vascular smooth muscle to the compound (Figure 6, p < 0.05, n = 6 in each group).

Fig. 6 - Relaxation to sodium nitroprusside in the canine coronary artery. Canine coronary arteries were exposed to either low or high intensity ultrasonic energy and contracted with prostaglandin F
2a (PGF
2a). When the PGF
2a contraction was stable, arteries were exposed to increasing concentrations of sodium nitroprusside. Data are presented as means ± SEM, n = 6
Isoproterenol (10
-9 - 10
-6 M) induced comparable concentration dependent relaxation in control and experimental coronary artery segments without endothelium. Either low or high intensity ultrasound energy output did not alter the maximal relaxation induced by isoproterenol or change the sensitivity of the vascular smooth muscle to the compound, as it was with sodium nitropruside (Figure 7, p < 0.05, n = 6 in each group).
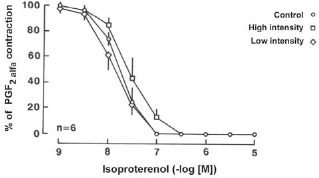
Fig. 7 - Relaxation to isoproterenol in the canine coronary artery. Canine coronary arteries were exposed to either low or high intensity ultrasonic energy and contracted with prostaglandin F
2a (PGF
2a). When the PGF
2a_ contraction was stable, arteries were exposed to increasing concentrations of isoproterenol. Data are presented as means ± SEM, n = 6
In patients with diffuse coronary artery disease, effective revascularization is impossible without improving arterial runoff. Coronary endarterectomy has been used to remove the obstructing plaque in occluded arteries with diffuse disease and enables them to be grafted [1,2,16]. However, endarterectomy is a technically challenging procedure and entails the risks of increased morbidity and mortality [1,3,16].
In this endarterectomy procedure, the surgeon elevates, dissects out and removes the core of the plaque, restoring a normal lumen. Techniques for distal coronary endarterectomy have included mechanical or manual methods as well as carbon dioxide gas and laser for dissection of the plaque [1,16,17]. Recently, application of intraarterial ultrasound has been shown to be feasible for use in recanalization of calcified atherosclerotic occlusions in vitro, in animals and in humans [4,18]. One of the important features of any device or technique used to treat obstructive arterial disease is its effect on arterial vasomotor behavior [18]. The use of ultrasonic waves as an adjunct to surgical endarterectomy can improve the effectiveness of the procedure by facilitating the removal of atheromatous plaque from the arterial wall. In this experiment the probe was placed into the vessel lumen. However, clinically the probe tip would be inserted between intima and the middle layer of the artery allowing the ultrasonic oscillations of the probe to help with the endarterectomy. Ultrasonic oscillations could allow the surgeon to perform the endarterectomy with less damage to the artery wall than present methods, facilitating the endarterectomy and reducing the risk of perforating the artery, especially in the presence of marked calcinosis.
It is well known that in the physical property analyses of one determined material it must be evaluated its magnitude and intensity. Thus, one third experimental group with application of the ultrasonic energy of low intensity for an extended time could be carried out. This detail was considered in the experiment design. The presented model was adopted based on the fact that energies applications for drawn out time it is not one practical usual in cardiac surgeries, which electric energies of low intensity and short duration is a kind of rule.
It has been shown that ultrasonic energy is capable of causing arterial relaxation and this relaxation is endothelium dependent and mediated by NO [19]. Controversially, in another study ultrasound-mediated relaxations have been shown to be endothelium independent and unlikely due to release of NO or other endothelium-derived substances (e.g., prostacyclin) [18]. These studies demonstrate the direct effect of ultrasound on the vessel wall. In our experiment, we studied the effect of ultrasound on the ability of the vessel to relax, and we showed that 25 W of ultrasonic energy impairs endothelium function but not smooth muscle function in canine epicardial coronary artery when the vessel was exposed to ultrasonic waves for 15 seconds.
Nowadays ultrasonic scalpels are commercially available and have already described advantages. The ultrasonic scalpel facilitates thoracoscopic internal mammary artery harvest and is expected to minimize hyperthermic damage of this artery [20,21].
Investigators recently demonstrated increased free blood flow from radial artery free grafts harvested using ultrasonic technology. Also, experimental canine internal artery sonication induces vasorelaxation almost completely by time-dependent endothelial nitric oxide and prostacyclin release, which appears unrelated to tissue heating or endothelial architectural disruption [22].
The left internal mammary artery dissection in a skeletonized fashion with an ultrasonic scalpel does not produce endothelial structural damage in it being similar to the one dissected with conventional methods. This permits its safe use, allowing us to benefit from the numerous advantages of arterial grafts usage in modern era coronary surgery [23].
Sternal perfusion increases soon after coronary bypass surgery, probably as a consequence of the healing process, but the source of perfusion for the harvest side remains unclear. Harvesting of internal thoracic arteries with an ultrasonic scalpel has no advantageous effects on postoperative sternal perfusion [24].
CONCLUSION
Results of the present study demonstrate that ultrasonic energy, delivered via a special endarterectomy probe causes energy dependent impairment in NO release from canine epicardial coronary artery. However, ultrasound energy does not alter endothelium-independent relaxation in canine coronary artery regardless of which energy level is used.
REFERENCES
1. Westaby S. Organ dysfunction after cardiopulmonary bypass: a systemic inflammatory reaction initiated by the extracorporeal circuit. Intensive Care Med. 1987;13(2):89-95. [
MedLine]
2. Edmunds LH Jr. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1998;66(5 Suppl):S12-6.
3. Royston D. The inflammatory response and extracorporeal circulation. J Cardiothorac Vasc Anesth. 1997;11(3):341-54. [
MedLine]
4. Moat NE, Shore DF, Evans TW. Organ dysfunction and cardiopulmonary bypass: the role of complement and complement regulatory proteins. Eur J Cardiothorac Surg. 1993;7(11):563-73. [
MedLine]
5. Frering B, Philip I, Dehoux M, Rolland C, Langlois JM, Desmonts JM. Circulating cytokines in patients undergoing normothermic cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1994;108(4):636-41. [
MedLine]
6. Nashef SA, Roques F, Hammill BG, Peterson ED, Michel P, Grover FL, et al. Validation of European System for Cardiac Operative Risk Evaluation (EuroSCORE) in North American cardiac surgery. Eur J Cardiothorac Surg. 2002;22(1):101-5. [
MedLine]
7. Vincent JL, Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793-800. [
MedLine]
8. Ungerleider RM. Effects of cardiopulmonary bypass and use of modified ultrafiltration. Ann Thorac Surg. 1998;65( 6 Suppl):S35-8.
9. Tetta C, D'Intini V, Bellomo R, Bonello M, Bordoni V, Ricci Z, et al. Extracorporeal treatments in sepsis: are there new perspectives? Clin Nephrol. 2003;60(5):299-304. [
MedLine]
10. Biglioli P, Cannata A, Alamanni F, Naliato M, Porqueddu M, Zanobini M, et al. Biological effects of off-pump vs. on-pump coronary artery surgery: focus on inflammation, hemostasis and oxidative stress. Eur J Cardiothorac Surg. 2003;24(2):260-9. [
MedLine]
11. Wang MJ, Chiu IS, Hsu CM, Wang CM, Lin PL, Chang CI, et al. Efficacy of ultrafiltration in removing inflammatory mediators in pediatric cardiac operations. Ann Thorac Surg. 1996;61(2):651-6. [
MedLine]
12. Wan S, Yim AP, Arifi AA, Lee TW, Huynh CH, DeSmet JM, et al. Can cardioplegia management influence cytokine responses during clinical cardiopulmonary bypass? Ann Thorac Cardiovasc Surg. 1999;5(2):81-5. [
MedLine]
13. Brunet S, Leblanc M, Geadah D, Parent D, Courteau S, Cardinal J. Diffusive and convective solute clearances during continuous renal replacement therapy at various dialysate and ultrafiltration flow rates. Am J Kidney Dis. 1999;34(3):486-92. [
MedLine]
14. Venkataraman R, Subramanian S, Kellum JA. Clinical review: extracorporeal blood purification in severe sepsis. Crit Care. 2003;7(2):139-45. [
MedLine]
15. Dinarello CA. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest. 1997;112(6 Suppl):321S-9S.
16. Janovich HB. The cytokine handbook. London:Academic Press;1991. p.5.
17. Butler J, Rocker GM, Westaby S. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1993;55(2):552-9. [
MedLine]
18. Butler J, Chong GL, Baigrie RJ, Pillai R, Westaby S, Rocker GM. Cytokine responses to cardiopulmonary bypass with membrane and bubble oxygenation. Ann Thorac Surg. 1992;53(5):833-8. [
MedLine]
19. Ohzato H, Yoshizaki K, Nishimoto N, Ogata A, Tagoh H, Monden M, et al. Interleukin-6 as a new indicator of inflammatory status: detection of serum levels of interleukin-6 and C-reactive protein after surgery. Surgery. 1992;111(2):201-9. [
MedLine]
20. Royston D, Fleming JS, Desai JB, Westaby S, Taylor KM. Increased production of peroxidation products associated with cardiac operations: evidence for free radical generation. J Thorac Cardiovasc Surg. 1986;91(5):759-66. [
MedLine]
21. Finn A, Naik S, Klein N, Levinsky RJ, Strobel S, Elliott M. Interleukin-8 release and neutrophil degranulation after pediatric cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1993;105(2):234-41. [
MedLine]
22. George JF. Invited letter concerning: cytokines and mechanisms of capillary leakage after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1993;106(3):566-7. [
MedLine]
23. Brasil LA, Gomes WJ, Salomão R, Buffolo E. Inflammatory response after myocardial revascularization with or without cardiopulmonary bypass. Ann Thorac Surg. 1998;66(1):56-9. [
MedLine]
24. Chenoweth DE, Cooper SW, Hugli TE, Stewart RW, Blackstone EH, Kirklin JW. Complement activation during cardiopulmonary bypass: evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med. 1981;304(9):497-503. [
MedLine]
25. Hill GE, Alonso A, Thiele GM, Robbins RA. Glucocorticoids blunt neutrophil CD11b surface glycoprotein upregulation during cardiopulmonary bypass in humans. Anesth Analg. 1994;79(1):23-7. [
MedLine]
26. Inaba H, Kochi A, Yorozu S. Suppression by methylprednisolone of augmented plasma endotoxin-like activity and interleukin-6 during cardiopulmonary bypass. Br J Anaesth. 1994;72(3):348-50. [
MedLine]
27. Dietzman RH, Casteda AR, Lillehei CW, Ersera, Motsay GJ, Lillehei RC. Corticosteroids as effective vasodilators in the treatment of low output syndrome. Chest. 1970;57(5):440-53. [
MedLine]
28. Niazi Z, Flodin P, Joyce L, Smith J, Mauer H, Lillehei RC. Effects of glucocorticosteroids in patients undergoing coronary artery bypass surgery. Chest. 1979;76(3):262-8. [
MedLine]
29. Fey K, Follette D, Livesay J, Nelson R, Bugyi H, DeLand, et al. Effects of membrane stabilization on the safety of hypothermic arrest after aortic cross-clamping. Circulation. 1977;56(3 Suppl):II143-7. [
MedLine]
30. Fosse E, Mollnes TE, Ingvaldsen B. Complement activation during major operations with or without cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1987;93(6):860-6. [
MedLine]
31. Brasil LA, Gomes WJ, Salomão R, Fonseca JHP, Branco JNR, E Buffolo. Uso de corticóide como inibidor da resposta inflamatória sistêmica induzida pela circulação extracorpórea. Rev Bras Cir Cardiovasc. 1999;14(3):254-68.
32. Jansen NJ, van Oeveren W, van den Broek L, Oudemans-van Straaten HM, Stoutenbeek CP, Joen MC, et al. Inhibition by dexamethasone of the reperfusion phenomena in cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1991;102(4):515-25. [
MedLine]
33. Liakopoulos OJ, Teucher N, Mühlfeld C, Middel P, Heusch G, Schoendube FA, et al. Prevention of TNFalpha-associated myocardial dysfunction resulting from cardiopulmonary bypass and cardioplegic arrest by glucocorticoid treatment. Eur J Cardiothorac Surg. 2006;30(2):263-70. [
MedLine]
34. Liakopoulos OJ, Schmitto JD, Kazmaier S, Bräuer A, Quintel M, Schoendube FA, et al. Cardiopulmonary and systemic effects of methylprednisolone in patients undergoing cardiac surgery. Ann Thorac Surg. 2007;84(1):110-8.
35. De Vriese AS, Colardyn FA, Philippé JJ, Vanholder RC, De Sutter JH, Lameire NH. Cytokine removal during continuous hemofiltration in septic patients. J Am Soc Nephrol. 1999;10(4):846-53. [
MedLine]
1. Bridgewater B, Steyn RS, Ray S, Hooper T. Minimally invasive aortic valve replacement through a transverse sternotomy: a word of caution. Heart. 1998;79(6):605-7. [
MedLine]
2. Szwerc MF, Benckart DH, Wiechmann RJ, Savage EB, Szydlowski GW, Magovern GJ Jr, et al. Partial versus full sternotomy for aortic valve replacement. Ann Thorac Surg. 1999;68(6):2209-13.
3. Kort S, Applebaum RM, Gross EA, Baumann FG, Colvin SB, Galloway AC, et al. Minimally invasive aortic valve replacement: echocardiographic and clinical results. Ann Thorac Surg. 2001;142(3):476-81.
4. Mächler HE, Bergmann P, Anelli-Monti M, Dacar D, Rehak P, Knez I, et al. Minimally invasive versus conventional aortic valve operations: a prospective study in 120 patients. Ann Thorac Surg. 1999;67(4):1001-5. [
MedLine]
5. Mulinari LA, Tyszka AL, Costa FDA, Carvalho RG, Silva Junior AZ, Giublin R, et al. Miniesternotomia: um acesso seguro para a cirurgia cardíaca. Rev Bras Cir Cardiovasc. 1997;12(4):335-9.
6. Aklog L, Adams DH, Couper GS, Gobezie R, Sears S, Cohn LH. Techniques and results of direct-access minimally invasive mitral valve surgery: a paradigm for the future. J Thorac Cardiovasc Surg. 1998;116(5):705-15. [
MedLine]
7. Chitwood WR Jr, Wixon CL, Elbeery JR, Moran JF, Chapman WH, Lust RM. Video-assisted minimally invasive mitral valve surgery. J Thorac Cardiovasc Surg. 1997;114(5):773-80.
8. Grossi EA, LaPietra A, Ribakove GH, Delianides J, Esposito R, Culliford AT, et al. Minimally invasive versus sternotomy approaches for mitral reconstruction: comparison of intermediate-term results. J Thorac Cardiovasc Surg. 2001;121(4):708-13. [
MedLine]
9. Morh FW, Falk V, Diegeler A, Walther T, van Son JA, Autschbach R. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg. 1998;115(3):567-74.
10. Stevens JH, Burdon TA, Peters WS, Siegel LC, Pompili MF, Vierra MA, et al. Port-access coronary artery bypass grafting: a proposed surgical method. J Thorac Cardiovasc Surg. 1996;111(3):567-73. [
MedLine]
11. Galloway AC, Shemin RJ, Glower DD, Boyer JH Jr, Groh MA, Kuntz RE, et al. First report of the Port Access International Registry. Ann Thorac Surg. 1999;67(1):51-6.
12. Mishra YK, Khanna SN, Wasir H, Sharma KK, Mehta Y, Trehan N. Port-access approach for cardiac surgical procedures: our experience in 776 patients. Indian Heart J. 2005;57(6):688-93. [
MedLine]
13. Greco E, Barriuso C, Castro MA, Fita G, Pomar JL. Port-Access cardiac surgery: from a learning process to the standard. Heart Surg Forum. 2002;5(2):145-9. [
MedLine]
14. Tatooles AJ, Pappas PS, Gordon PJ, Slaughter MS. Minimally invasive mitral valve repair using the da Vinci robotic system. Ann Thorac Surg. 2004;77(6):1978-82.
15. Reichenspurner H, Boehm D, Reichart B. Minimally invasive mitral valve surgery using three-dimensional video and robotic assistance. Semin Thorac Cardiovasc Surg. 1999;11(3):235-43. [
MedLine]
16. Reichenspurner H, Boehm DH, Gulbins H, Schulze C, Wildhirt S, Welz A, et al. Three-dimensional video and robot-assisted port-access mitral valve operation. Ann Thorac Surg. 2000;69(4):1176-81.
17. Jatene FB, Pego-Fernandes PM, Assad RS, Dallan LA, Hueb W, Arbulu HEVD, et al. Cirurgia de revascularização do miocárdio minimamente invasiva: resultados com o uso da videotoracoscopia e do estabilizador de sutura. Rev Bras Cir Cardiovasc. 1997;12(3):233-8.
18. Souto GLL, Tinoco RC, Tinoco ACA, Caetano CS, Souza JB, Paula AG, et al. Ligadura videotoracoscópica da persistência do canal arterial. Rev Bras Cir Cardiovasc. 2000;15(2):154-9.
19. Salerno PR, Jatene MB, Santos MA, Ponce F, Bosísio IBJ, Fontes VF, et al. Fechamento de canal arterial por minitoracotomia: técnica e resultados. Rev Bras Cir Cardiovasc. 2000;15(3):234-7.
20. Grossi EA, Galloway AC, LaPietra A, Ribakove GH, Ursomanno P, Delianides J, et al. Minimally invasive mitral valve surgery: a 6-year experience with 714 patients. Ann Thorac Surg. 2002;74(3):660-3.
21. Woo YJ, Nacke EA. Robotic minimally invasive mitral valve reconstruction yields less blood product transfusion and shorter length of stay. Surgery. 2006;140(2):263-7. [
MedLine]
22. Navia JL, Atik FA, Grimm RA, Garcia M, Vega PR, Myhre U, et al. Minimally invasive left ventricular epicardial lead placement: surgical techniques for heart failure resynchronization therapy. Ann Thorac Surg. 2005;79(5):1536-44.
23. Argenziano M, Oz MC, DeRose JJ Jr, Ashton RC Jr, Beck J, Wang F, et al. Totally endoscopic atrial septal defect repair with robotic assistance. Heart Surg Forum. 2002;5(3):294-300. [
MedLine]
24. Torracca L, Ismeno G, Quarti A, Alfieri O. Totally endoscopic atrial septal defect closure with a robotic system: experience with seven cases. Heart Surg Forum. 2002;5(2):125-7. [
MedLine]
25. Casselman FP, La Meir M, Jeanmart H, Mazzarro E, Coddens J, Van Praet F, et al. Endoscopic mitral and tricuspid valve surgery after previous cardiac surgery. Circulation. 2007;116(11 Suppl):I270-5. [
MedLine]
1. Loop FD. Resurgence of coronary artery endarterectomy. J Am Coll Cardiol. 1988;11(4):712-3. [
MedLine]
2. Walley VM, Byard RW, Keon WJ. A study of the sequential morphologic changes after manual coronary endarterectomy. J Thorac Cardiovasc Surg. 1991;102(6):890-4. [
MedLine]
3. Keon WJ, Masters RG, Koshal A, Hendry P, Farrell EM. Coronary endarterectomy. An adjunct to coronary artery bypass grafting. Surg Clin North Am. 1988;68(3):669-78. [
MedLine]
4. Demer LL, Ariani M, Siegel RJ. High intensity ultrasound increases distensibility of calcific atherosclerotic arteries. J Am Coll Cardiol. 1991;18(5):1259-62. [
MedLine]
5. Siegel RJ, Fishbein MC, Forrester J, Moore K, DeCastro E, Daykhovsky L, et al. Ultrasonic plaque ablation. A new method for recanalization of partially or totally occluded arteries. Circulation. 1988;78(6):1443-8. [
MedLine]
6. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373-6. [
MedLine]
7. Ignarro LJ. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989;3(1):31-6. [
MedLine]
8. Bassenge E, Busse R. Endothelial modulation of coronary tone. Prog Cardiovasc Dis. 1988;30(5):349-80. [
MedLine]
9. Vanhoutte PM, Shimokawa H. Endothelium-derived relaxing factor and coronary vasospasm. Circulation. 1989;80(1):1-9. [
MedLine]
10. Sneddon JM, Vane JR. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc Natl Acad Sci USA. 1988;85(8):2800-4.
11. Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2(8567):1057-8. [
MedLine]
12. Azuma H, Ishikawa M, Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986;88(2):411-5. [
MedLine]
13. Furlong B, Henderson AH, Lewis MJ, Smith JA. Endothelium-derived relaxing factor inhibits in vitro platelet aggregation. Br J Pharmacol. 1987;90(4):687-92. [
MedLine]
14. Pearson PJ, Schaff HV, Vanhoutte PM. Acute impairment of endothelium-dependent relaxations to aggregating platelets following reperfusion injury in canine coronary arteries. Circ Res. 1990;67(2):385-93. [
MedLine]
15. Pearson PJ, Schaff HV, Vanhoutte PM. Long-term impairment of endothelium-dependent relaxations to aggregating platelets after reperfusion injury in canine coronary arteries. Circulation. 1990;81(6):1921-7. [
MedLine]
16. Livesay JJ, Cooley DA, Hallman GL, Reul GJ, Ott DA, Duncan JM, et al. Early and late results of coronary endarterectomy. Analysis of 3,369 patients. J Thorac Cardiovasc Surg. 1986;92(4):649-60. [
MedLine]
17. Livesay JJ. Laser technique for coronary endarterectomy. Adv Cardiol. 1988;36:54-61. [
MedLine]
18. Fischell TA, Abbas MA, Grant GW, Siegel RJ. Ultrasonic energy. Effects on vascular function and integrity. Circulation. 1991;84(4):1783-95. [
MedLine]
19. Chokshi SK, Rongione AJ, Freeman I, Gal D, Grunwald AM, Alliger H. Ultrasonic energy produces endothelium-dependent vasomotor relaxation in vitro. Circulation. 1989;80(suppl II):II-565.
20. Ohtsuka T, Wolf RK, Hiratzka LF, Wurnig P, Flege JB Jr. Thoracoscopic internal mammary artery harvest for MICABG using the Harmonic Scalpel. Ann Thorac Surg. 1997;63(6 Suppl):S107-9. [
MedLine]
21. Chen Y, Luo X, Shi W, Zhou Z. The application and development of ultrasonic scalpel. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2005;22(2):377-80. [
MedLine]
22. Maruo A, Hamner CE, Rodrigues AJ, Higami T, Greenleaf JF, Schaff HV. Nitric oxide and prostacyclin in ultrasonic vasodilatation of the canine internal mammary artery. Ann Thorac Surg. 2004;77(1):126-32. [
MedLine]
23. Lima Cañadas PP, Cañas AC, Orradre Romeo JL, Rubio Martínez CI, López Almodóvar LF, Calleja Hernández M. Endothelium histological integrity after skeletonized dissection of the left internal mammary artery with ultrasonic scalpel. Interact Cardiovasc Thorac Surg. 2005;4(3):160-2. [
MedLine]
24. Pektok E, Cikirikcioglu M, Engin C, Daglioz G, Ozcan Z, Posacioglu H. Does harvesting of an internal thoracic artery with an ultrasonic scalpel have an effect on sternal perfusion? J Thorac Cardiovasc Surg. 2007;134(2):442-7. [
MedLine]







 All scientific articles published at bjcvs.org are licensed under a Creative Commons license
All scientific articles published at bjcvs.org are licensed under a Creative Commons license