We tested the hypothesis that TMLR combined with intramyocardial injection of BMC is safe, and may help increase the functional capacity of patient with refractory angina.
Nine patients (eight men), 65±5 years old, with refractory angina for multivessel disease and previous myocardial revascularization procedures (CABG/PCI), not candidates for another procedure due to the extension of the disease were enrolled. TMLR (11±3 laser drills) was performed via a limited thoracotomy using a CO
Heart Laser System. BMC were obtained immediately prior to surgery, and the lymphomonocytic fraction separated by density gradient centrifugation. During surgery, 5mL containing approximately 1.9±0.3x108 BMC were delivered by multiple injections in the ischemic myocardium. Before (B) and 6 months (6M) after the procedure, patient underwent clinical evaluation and myocardial perfusion assessment by cardiac magnetic resonance imaging (MRI) during pharmacological stress with dypiridamole.
No major complications or deaths occurred during the procedure. One patient died after 2 years (non cardiac cause).There was a reduction in the ischemic score as assessed by MRI from 1.64±0.10 (B) to 0.88±0.09 (6M) (P=0.01). Clinically, there was a reduction in functional class of angina from 3.7±0.2 (B) to 1.3±0.2 (6M) (P<0.0001).
In this initial experience, the combined strategy of TMLR plus cell therapy appeared to be safe, and may have synergistically acted to reduce myocardial ischemia, with clinically relevant improvement in functional capacity. Provided these data are confirmed in a larger, randomized, controlled trial with longer follow-up, this strategy could be used as a novel therapeutic option for treating pt with refractory angina.
É descrita uma proposição cirúrgica para o tratamento de pacientes com doença arterial coronária (DAC) terminal, não mais passíveis de revascularização miocárdica convencional. Constitui-se na revascularização transmiocárdica com raios laser (RTML), associada ao emprego de células progenitoras hematopoiéticas autólogas (CPH).
Nove pacientes (oito homens), 65±5 anos, com as características supracitadas foram submetidos ao procedimento combinado. Além da avaliação clínica, o protocolo incluiu o estudo da perfusão miocárdica através da ressonância cardíaca (RMC) sob estresse farmacológico, antes e seis meses após a intervenção cirúrgica. Procedeu-se à RMTL através de minitoracotomia esquerda e utilização de laser de CO
, com média de 11±3 tiros por paciente. As CPH foram obtidas por punção medular, seguindo-se sua injeção direta (1,9±0,3x108 células/paciente) em múltiplas áreas do miocárdio isquêmico.
Não ocorreram óbitos ou complicações imediatas decorrentes dos procedimentos. Um paciente faleceu no segundo ano de pós-operatório, de causa não cardíaca (choque séptico). O seguimento clínico pós-operatório desses pacientes revelou redução significativa da classe funcional de angina de 3,7±0,2 para 1,3±0,2 (p<0,0001). Também se verificou redução estatística do índice isquêmico do ventrículo esquerdo (VE) avaliado pela RMC de 1,64±0,10 para 0,88±0,09 (p=0,01).
A associação da terapia celular com a RTML demonstrou-se segura nessa experiência inicial. Caso confirmado esse sinergismo em estudos mais abrangentes, com melhora da angina e redução documentada da isquemia miocárdica, passamos a contar com uma nova possibilidade de tratamento alternativo para esse grave grupo de pacientes.
INTRODUCTION
The Transmyocardial Laser Revascularization Surgery (TMLR) is a therapeutic option for patients with end-stage ischemic coronary artery disease due to the advancement of atherosclerotic disease [1]. These patients are not eligible for conventional CABG or procedures performed through percutaneous intervention. Several studies have attempted to establish the activity mechanisms of the laser, since one of the main causes of improvement in myocardial perfusion of these patients has been attributed to the actions of angiogenic cytokines, due to the inflammatory process triggered by the laser [2,3].
Recently, cellular therapy emerged as a therapeutic strategy for tissue repair in cardiovascular medicine [4,5]. In experimental animal models, it was shown that bone marrow cells have the capacity to differentiate between contractile cells or blood vessels in ischemic tissues [6,7]. After laboratory documentation regarding the therapeutic potential of these cells, clinical trials for coronary artery disease (CAD) were initiated [8,9]. Clinical trial compilations indicate the safety of the procedure and its statistically significant efficacy, although with marginal clinical relevance [10,11]. Facing the angiogenic potential of these two therapeutic modalities, a synergic effect may be obtained with subsequent optimization gain of the tissue perfusion was suggested as a result of a combination between TMLR and cellular therapy in patients with refractory angina. In fact, the previous experience of this team has shown the feasibility of the combination of both techniques in patients with refractory angina, with subsequent increase of the myocardial perfusion and improvement of angina functional class [12].
The aim of this study is to verify that the TMLR associated with cellular therapy is feasible and safe, and also that the angiogenic synergic effect of both therapies can provide benefits to these patients in terms of angina relief and the myocardial ischemia reduction.
METHOD
Population Study
Between August 2004 and December 2006, nine patients (eight men) with a mean age of 65±5 years and with refractory angina (even after high-quality pharmacological therapy) and CAD were operated in our service. The established involvement criteria were: age between 18 and 75 years; refractory angina in spite of maximally tolerated clinical therapy; myocardial ischemia objectively documented by noninvasive methods; and coronariography with multiarterial obstructive pattern. Three patients presented with angina Class III, six presented with Class IV, and all had been previously revascularized and were not candidates for reintervention because of diffused coronary artery disease. This is a phase I, non-randomized, non-prospective open study approved by the Institution's ethics committee, as well as by all patients who agreed to participate in the study and who signed an informed consent form.
Surgical Procedure
A TMLR was performed through left anterior mini-thoracotomy and a CO2 laser (PLC Medical Systems, Milford, MA) was used, with a mean of 11±3 shots per patient that were distributed in the anterior, lateral and inferior walls of the heart (Figure 1A). The autologous hematopoietic progenitor cells (AHPC) were obtained during a bone marrow puncture performed during the same surgical procedure and immediately after administering the anesthesia. About 100ml of bone marrow liquid were extracted from the posterior iliac crest, and their lymphomononuclear fraction was separated from the density gradient using the Ficoll-Paque Plus (Amersham Biosciences, Piscataway, NJ) system. Thus, the separated cells were re-suspended in saline solution at a final volume of 5-mL, and containing 1.9±0.3x108 AHPC (CD34+=1.8±0.3%). The injection into the myocardium was directly performed in its several ischemic areas around the channels that were made by the laser shots (Figure 1B). Next, the final stage of the surgical procedure was performed in a routine manner, and the patients were sent to the cardiac care unit.
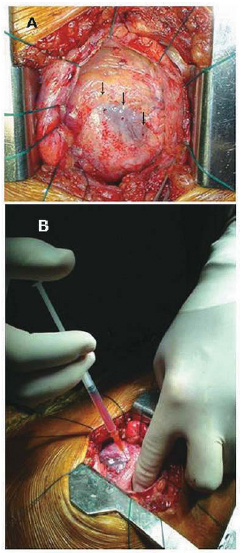
Fig. 1 - A: Channels created by laser shots in the anterior wall of LV (arrows). B: Myocardial injection of AHPC combined to TML
T
Myocardial perfusion study by cardiac resonance
Along with a clinical evaluation, the patients underwent a standardized study of myocardial perfusion by cardiac resonance (CMR) (1.5 T scanner - Sgna CV/i, GE Medical Systems, Waukesha, WI), with pharmacological stress in the preoperative and also after six months, as previously described [12,13].
Statistical analysis
The continuous variables are presented as mean ± standard deviation. Student's two-tailed t-test was used for comparisons between the pre- and postoperative periods. The level of statistical significant was defined as a p-value < 0.05. The statistical calculations were performed using the SPSS 13.0 software for Windows (SPSS Inc., Chicago, IL).
RESULTS
Clinical evolution
There were no deaths or immediate complications from the procedure. One death 2 years after the procedure occurred as a result of septic shock (non-cardiac origin). The postoperative clinical follow-up of these patients showed significant reduction of the angina functional class from 3.7± 0.2 (preoperative) to 1.3± 0.2 (six-month postoperative), p< 0.0001, with all patients improving by at least two angina functional classes in six months of follow-up (Figure 2). Among the eight surviving patients, five are in class I, and three are in class II. There was also a reduction in the use of medication (particularly the short-acting nitrates).
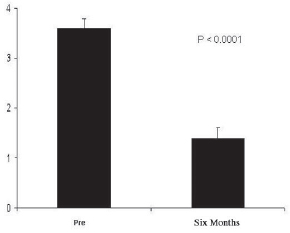
Fig. 2 - Angina CCS class six months before and after the procedure. We observed a significant reduction in angina class from 3.7±0.2 (preoperative) to 1.3±0.2 (six months postoperative), p< 0.0001
In addition to clinical observation, all patients underwent postoperative CMR in order to evaluate and record the myocardial perfusion six months after the procedure. The CMR performed in the nine patients showed a significant reduction in the ischemic score of the left ventricle, decreasing from 1.64± 0.10 (pre-operative) to 0.88± 0.09 (six-month postoperative), p=0.01 (Figure 3). There was no significant alteration in the left ventricle ejection fraction, which was between 0.54±0.09 and 0.59±0.07, p=0.41. Figure 4 illustrates the kind of image obtained by CMR during the study of myocardial perfusion during pharmacological stress.
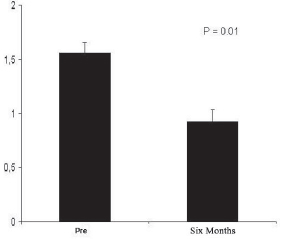
Fig. 3 - Ischemic score of LV determined by CMR before and six months after the procedure. We observed a significant reduction in the ischemic score of the ischemic index of the left ventricle, decreasing from 1.64±0.10 (preoperative) to 0.88±0.09 (six months postoperative), p=0.01
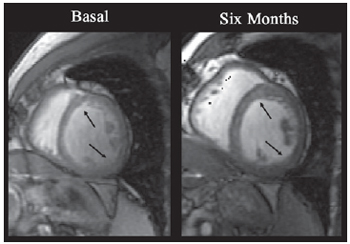
Fig. 4 - Images obtained by CMR from the short axis of LV. The arrows indicate myocardial segments with clear increase of contractility. An increase of systolic parietal thickening after procedure was noted when compared to base levels
The discussion of this article directly involves the possible methods of TMLR and cellular therapy in the ischemic myocardium and their possible therapeutic interaction. Randomized studies with the use of TMLR have shown the beneficial effects of this procedure: the decrease of the angina score in patients with severe and diffuse CAD {14-16]. However, the true mechanism of action remains unclear. The initial purpose was to achieve, as with the reptile heart, myocardial perfusion through channels that would bring arterial blood directly from the left ventricle toward the myocardium. However, this initial plan was not completed [17].
Among the possible mechanisms of action of TMLR, an eventual placebo effect can be included that, for its turn, difficultly may remain over several years after the procedure. Cardiac denervation using laser rays would be another possibility. The presence of ventricular receptors, as well as sympathetic afferent fibers (which send painful cardiac stimuli into the brain) would corroborate with this hypothesis. However, this event tends to be temporary because there is evidence of re-innervation after six months [18,19]. Another hypothesis may come from the destruction of the ischemic myocardium. We still must ask if the extension of the laser shots on the myocardial surface justifies this possibility. We can take the example of the use of holmium (YAG laser), which is admittedly more abrasive than the CO
2 laser. If we consider the performance of 40 laser shots (currently, this number is considerably less than before), the mean thickness of the LV wall (10mm) and the knowledge of the lesion area of each laser shot (4.5mm2) [20], we may conclude that the total volume of the destroyed myocardial tissue is around 1.8mm
3.
In terms of the tissue density, this number corresponds to only 2 grams of muscle. If the CO
2 laser had been used, the volume of destroyed myocardium would be the half of this number. If we consider that the mean left ventricular mass index corresponds to 76±13g/m
2 of the BMI in men and 66±11g/m
2 in women [21], we verify that the treatable muscle mass is 80 to 113g in men and 62.5 to 88g in women (taking into account that the one third of this mass corresponds to the interventricular septum that is out of the laser target range). Thus, the myocardium volume destroyed by TMLR therapy, depending on the gender and the body mass of the patient, would correspond with 1.7% to 3.2% of the total of treatable muscle, which is an insignificant quantity to explain angina relief.
Recently, a new mechanism for the action of TMLR was proposed. The hypothesis is based on the redistribution of left ventricular wall stress areas through the lasered creation of transmyocardial fibrous scars, which may penetrate through the several muscular layers that cicle the left ventricular cavity [22]. The more superficial muscle fibers are disposed in oblique position, while those in the median layer are circumferential, and the internal muscle fibers have longitudinal arrangement [23]. Hearts with diffuse ischemia and preserved retractile function may have multiple microscopic areas of undetectable hypokinesia, which could generate other compensatory areas of hyperkinesia [24]. In these hyperkinetic areas, there would be an increase of oxygen demand and the accumulation of metabolic residue. Due the fact that these areas have limited blood flow and oxygenation, this compensatory mechanism could induce angina. Transmyocardial fibrosis decreased the tension from the simultaneous contraction of the muscle fibers that are distributed in the different aforementioned directions. Thus, the left ventricle stress wall would be divided by "mini-tendons" on all layers between the endocardium and the epicardium, whose sustentance would redistribute the interfascicular tension and which would reduce the oxygen demand. The effects of TMLR in the angina reduction have been attributed to angiogenesis, although there is no evidence that it is connected to a specific action of the laser [25,26]. It is unquestionable that the increase in the vascular density in the areas around the channels was caused by the laser shots [17]. However, we can calculate that, after making of 40 channels by TMLR, only 17.4% to 31.5% of the myocardial treatable tissue is able to microscopically develop neovascularization (depending on the gender and the body surface of the patient) [22].
However, the fact that this neovascularization does not expand past end of the contiguous territories [27,28]. Several studies indicate an improvement in myocardial perfusion after TMLR, especially those performed by thallium scintigraphy [16,29]. The question still remains about how this new revascularization could occur in territories that are already fibrous, and that are recognizable sites with a low availability of oxygen after myocardial infarction [18]. The cellular therapy aims to recover the loss of adult heart cells, "refilling" the fibrous region with new contractile cells and/or promoting angiogenesis [30]. Hence, the presence of sustained ischemia is a fundamental factor in therapeutic success. The multipotent adult progenitor cells are similar to embryogenic stem cells in that they maintain the differentiation and cloning potential in several cell lines, beyond the extensive proliferation capacity [31]. These cells are also advantageous because they have fewer immunological and ethical problems, aside from their immediate use in cell therapy. Although there are such cells in several tissues (such as, for example, bone marrow, blood and liver), they are found in lesser quantity in adults [32]. The typical example of adult stem cells are hematopoietic progenitor cells, which have a hierarchically organized system with multi-potent stem cells with the capacity for self-renovation beyond the capacity to generate all cell types of the immune system and the blood.
Despite intense studies aiming to decipher the molecular scheme of line exchange in the hematopoietic system, the mechanism that determines the transition in the destiny of adult stem cells is still not clear. Studies suggest that the adult stem cells, including the hematopoietic progenitor cells, maintain a differentiated grade of "plastic development," which allows for their differentiation according to the cell line, organs or tissues that are with them or surrounding them [33-35]. However, this classical concept of restricted stem-cell differentiation in specific lines of the patients' own organs is currently questioned. The aim of this study clearly indicates the possibility of the appearance of mechanisms that can improve perfusion and, consequently, the symptomatology of this select range of patients with coronary artery disease. Although controversial, either the attempt at transdifferentiation of autologous endothelial progenitor cells, when transplanted in the ischemic myocardium for therapeutic revascularization [36], or the action of cytokines and growth factors locally applied after TMLR or associated to cells injection [37], or both, may have acted synergically, allowing the angiogenesis and subsequent functional improvement that showed the importance of the micro-environment in obtaining the desired therapeutic effect through cell therapy [38].
This possibility of synergism in cell therapy procedures and TMLR occurred in one patient of our study. The patient was a 74-year-old patient with refractory angina who was treated with bone marrow autologous cells combined with TMLR. The basic CMR revealed extensive areas of myocardial ischemia associated with poor contractile performance of the LV. Eleven CO
2 laser shots and a myocardial injection of 5ml suspension with 21.5X10
6 bone marrow cells per ml were performed. Six months after the procedure, another TMLR showed complete resolution of the perfusion defects and accentuated improvement of LV contractile function [12].
This study showed the feasibility of the combination of two different strategies (TMLR associated with cell therapy) in highly symptomatic patients with coronary artery disease that was non-responsive to medical treatment and in which all existing therapeutic resources had already been exhausted. As in all new propositions, we must encourage other investigations to try to prove or disprove the points herein described. New research lines must be developed that can definitively establish the safety, the efficacy, and both the positive and negative potential effects related to the procedure.
CONCLUSION
In this initial study (Phase I), the association of TMLR with intramyocardial application of stem cells was feasible and safe, and the synergism of both therapies may have contributed to the reduction of myocardial ischemia and to the angina improvement in this group of patients. If this synergism were confirmed in more comprehensible studies, with more patients (double-blind, randomized and controlled by placebo), we may have an alternative method for the treatment of this severe group of patients, which is currently highly restricted by refractory angina that lacks available therapeutic alternatives.
REFERENCES
1. Frazier OH, March RJ, Horvath KA. Transmyocardial revascularization with a carbon dioxide laser in patients with end-stage coronary artery disease. N Engl J Med. 1999;341(14):1021-8. [
MedLine]
2. Bridges CR. Angiogenesis in myocardial laser "revascularization". Herz. 2000;25(6):579-88. [
MedLine]
3. Fuchs S, Baffour R, Vodovotz Y, Shou M, Stabile E, Tio FO, et al. Laser myocardial revascularization modulates expression of angiogenic, neuronal, and inflammatory cytokines in a porcine model of chronic myocardial ischemia. J Card Surg. 2002;17(5):413-24. [
MedLine]
4. Cannon RO 3rd, Dunbar CE. BM-derived cell therapies for cardiovascular disease. Cytotherapy. 2007;9(4):305-15. [
MedLine]
5. Renault MA, Losordo DW. Therapeutic myocardial angiogenesis. Microvasc Res. 2007;74(2-3):159-71. [
MedLine]
6. Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98(18):10344-9. [
MedLine]
7. Yang ZJ, Ma DC, Wang W, Xu SL, Zhang YQ, Chen B, et al. Neovascularization and cardiomyocytes regeneration in acute myocardial infarction after bone marrow stromal cell transplantation: comparison of infarct-relative and noninfarct-relative arterial approaches in swine. Clin Chim Acta. 2007;381(2):114-8. [
MedLine]
8. Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115(25):3165-72. [
MedLine]
9. Briguori C, Reimers B, Sarais C, Napodano M, Pascotto P, Azzarello G, et al. Direct intramyocardial percutaneous delivery of autologous bone marrow in patients with refractory myocardial angina. Am Heart J. 2006;151(3):674-80. [
MedLine]
10. Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50(18):1761-7. [
MedLine]
11. Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167(10):989-97. [
MedLine]
12. Gowdak LH, Schettert IT, Rochitte CE, Lisboa LA, Dallan LA, César LA, et al. Cell therapy plus transmyocardial laser revascularization for refractory angina. Ann Thorac Surg. 2005;80(2):712-4. [
MedLine]
13. Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, et al. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. Eur Heart J. 2004;25(21):1940-65. [
MedLine]
14. Horvath KA. Results of prospective randomized controlled trials of transmyocardial laser revascularization. Heart Surg Forum. 2002;5(1):33-9.
15. Allen KB, Dowling RD, Fudge TL, Schoettle GP, SelingerSL, Gangahar DM, et al. Comparison of transmyocardial revascularization with medical therapy in patients with refractory angina. N Engl J Med. 1999;341(14):1029-36. [
MedLine]
16. Allen KB, Dowling RD, Schuch DR, Pfeffer TA, Marra S, Lefrak EA, et al. Adjunctive transmyocardial revascularization: five-year follow-up of a prospective, randomized trial. Ann Thorac Surg. 2004;78(2):458-65.
17. Dallan LA, Lisboa LA, Abreu-Filho CAC, Cabral RH, Platania F, Dallan LAP, et al. Análise direcional do fluxo sangüíneo miocárdico após revascularização transmiocárdica com laser de CO2: estudo através da ressonância magnética com imagens de gradiente ultra-rápido. Rev Bras Cir Cardiovasc. 2002;17(2):1-7.
18. Sun Y, Kiani MF, Postlethwaite AE, Weber KT. Infarct scar as living tissue. Basic Res Cardiol. 2002;97(5):343-7. [
MedLine]
19. Minisi AJ, Topaz O, Quinn MS, Mohanty LB. Cardiac nociceptive reflexes after transmyocardial laser revascularization: implications for the neural hypothesis of angina relief. J Thorac Cardiovasc Surg. 2001;122(4):712-9. [
MedLine]
20. Fisher PE, Khomoto T, DeRosa CM, Spotnitz HM, Smith CR, Burkhoff D. Histologic analysis of transmyocardial channels: comparison of CO2 and holmium:YAG lasers. Ann Thorac Surg. 1997;64(2):466-72. [
MedLine]
21. Echo by web: left ventricular geometry. Disponível em: http://www.echobyweb.com/htm_level3_outofstructure/formulas & calculations/lv_geometry_eng.htm. Acesso em: 28/ 01/2008.
22. Cardarelli M. A proposed alternative mechanism of action for transmyocardial revascularization prefaced by a review of the accepted explanations. Tex Heart Inst J. 2006;33(4):424-6. [
MedLine]
23. Anderson RH, Becker AE, Allwork SP. Cardiac anatomy: an integrated text and colour atlas. London:Gower Medical;1980. p.5.2-5.26.
24. Braunwald E. Heart disease: a textbook of cardiovascular medicine. Philadelphia:WB Saunders;1980. p.429-78.
25. Bridges CR. Myocardial laser revascularization: the controversy and the data. Ann Thorac Surg. 2000;69(2):655-62. [
MedLine]
26. Bridges CR. Angiogenesis in myocardial laser "revascularization". Herz. 2000;25(6):579-88. [
MedLine]
27. Kohmoto T, DeRosa CM, Yamamoto N, Fisher PE, Failey P, Smith CR, et al. Evidence of vascular growth associated with laser treatment of normal canine myocardium. Ann Thorac Surg. 1998;65(5):1360-7. [
MedLine]
28. Mueller XM, Tevaearai HT, Genton CY, Chaubert P, von SegesserLK. Are there vascular density gradients along myocardial laser channels? Ann Thorac Surg. 1999;68(1):125-9.
29. Lange RA, Hillis LD. Transmyocardial laser revascularization. N Engl J Med. 1999;341(14):1075-6. [
MedLine]
30. Dallan LAD, Lisboa LA, Oliveira AS. Possibilidade do transplante celular na miocardiopatia terminal. In: Buffolo E, ed. Insuficiência cardíaca: uma visão mecanicista. São Paulo:Atheneu;2005. p.105-27.
31. Lisboa LAF, Dallan LAO, Oliveira SA. Implante miocárdico de células-tronco na miocardiopatia isquêmica. In: Oliveira SA, Lisboa LAF, Dallan LAO, eds. Cirurgia cardiovascular (Série Colégio Brasileiro de Cirurgiões). vol 3. São Paulo:Atheneu;2005. p.75-82.
32. Korbling M, Estrov Z. Adult stem cells for tissue repair: a new therapeutic concept? N Engl J Med. 2003;349(6):570-82. [
MedLine]
33. Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105(7):829-41. [
MedLine]
34. Quesenberry PJ, Colvin GA, Lambert JF. The chiaroscuro stem cell: a unified stem cell theory. Blood. 2002;100(13):4266-71. [
MedLine]
35. Graf T. Differentiation plasticity of hematopoietic cells. Blood. 2002;99(9):3089-101. [
MedLine]
36. Kawamoto A, Tkebuchava T,Yamaguchi J, Nishimura H, Yoon YS, Milliken C, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 2003;107(3):461-8. [
MedLine]
37. Liu Y, Guo J, Zhang P, Zhang S, Chen P, Ma K. Bone marrow mononuclear cell transplantation into heart elevates the expression of angiogenic factors. Microvasc Res. 2004;68(3):156-60. [
MedLine]
38. Oliveira SA, Gowdak LH, Buckberg G, Krieger JE; RESTORE Group. Cell biology, MRI and geometry: insight into a microscopic/macroscopic marriage. Eur J Cardiothorac Surg. 2006;29(Suppl 1):S259-65. [
MedLine]




 All scientific articles published at bjcvs.org are licensed under a Creative Commons license
All scientific articles published at bjcvs.org are licensed under a Creative Commons license