INTRODUCTION
Acute coronary syndromes, including stable angina, myocardial infarction, both with and without ST segment elevation and sudden death are responsible for more than two millions of hospitalizations and 30% of all deaths in the United States [1,2]. In Brazil, cardiovascular diseases are the main cause of death; responsible for one-third of all deaths in the country. According to data based on Death reported to the DATASUS database of the Brazilian Heath Ministry, the coefficient of death by cardiovascular diseases is approximately 442 per 100,000 inhabitants [3]. These clinical entities are the consequence of atherosclerotic plaque rupture or significant fissure, with platelet aggregation and the formation of subocclusive or occlusive thrombi [1,2]. The rupture of this plaque exposes its central fat content and causes platelet adhesion and activation of the coagulation cascade [1,2]. Activated platelets release diverse vasoactive substances, including thromboxane A2 (TXA) and adenosine diphosphate (ADP) that cause platelet adhesion and primary hemostasis [1,2]. Secondary hemostasis occurs as a result of the conversion of the fibrinogen to fibrin mediated by thrombin and subsequent stabilization of platelet aggregation [1,2].
In respect to atherothrombotic phenomena, the most common platelet inhibitor utilized worldwide is aspirin [4,5]. Many studies have demonstrated the efficacy of aspirin in primary and secondary prevention of myocardial infarction, strokes and death due to cardiovascular diseases [4,5]. A recent metanalysis revealed that, among patients with high vascular risk, therapy with aspirin was associated with a reduction of 34% of non-fatal myocardial infarction, 25% of non-fatal strokes and 18% of all causes of death [6].
Atherothrombosis is thus a complex physiological process and the absolute risk of recurrent vascular events among patients who take aspirin remains high; estimated at between 8% and 18% over two years [1,2]. This suggests that the antiplatelet effects of aspirin may not be homogeneous for all patients and that multiple therapeutic agents may be necessary to adequately blockade platelet function [1,2].
Measurement of platelet aggregation, platelet activation and bleeding time confirm the variability of the antithrombotic responses in patients treated with aspirin [7]. Prospective clinical studies have demonstrated that the reduction in the response to therapy using aspirin is associated with an increase in risk of atherothrombotic events [8,9]. These observations contribute to consolidate the concept of aspirin resistance, that is, the failure of aspirin to produce a satisfactory biological response, including platelet inhibition or the incapacity of aspirin to prevent atherothrombotic events [1,2]. Moreover, given the prevalence of cardiovascular diseases, identification and clinical analysis of patients insensitive to the antiplatelet effects of aspirin may contribute to the search for alternatives that achieve appropriate levels of platelet inhibition.
ASPIRIN
Mechanism of action
When the intimal layer of blood vessels is injured, for example, after injury or rupture of atherosclerotic plaque the subendothelial collagen and von Willebrand factors enter the blood circulation. Platelets adhere to the subendothelial collagen and von Willebrand factors by means of glycoproteins Ia/IIa and glycoproteins Ib/V/IX receptors. Platelet adhesion stimulates platelet activation, which causes changes in the shape of platelets and the release of high quantities of calcium to enter platelets [10,11].
The increase of free ionized calcium concentrations in platelets has several consequences. First, it induces a conformational change in the platelet receptors of the glycoprotein IIb/IIIa, allowing platelets to bind to adhesive proteins in the blood circulation, including fibrinogen. Second, it catalyzes the release of active molecules of platelet granules into the blood circulation, where they can bind to the surface receptors of adjacent platelets, stimulating their activation. Third, it promotes the action of phospholipase A2, which is responsible for the production of arachidonic acid [10,11].
Platelet arachidonic acid is converted to TXA2, in a reaction catalyzed by the cyclooxygenase-l enzyme (COX-1) forming prostaglandin G2/H2 and thromboxane synthase to form TXA2. TXA2 increases the expression of fibrinogen receptors on the platelet surface and is released into the circulation, where there is interaction with thromboxane receptors on the surface of adjacent platelets, stimulating their respective activation. TXA2 also acts synergically with other products released by the activated platelets, such as ADP, fibrinogen and Factor V, intensifying platelet activation. Moreover, TXA2 is a potent vasoconstrictor [10,11].
Aspirin (acetylsalicylic acid) reduces platelet activation through the irreversible acetylation of COX-1 and, thus, reduces the production of TXA2 by platelets. COX-1 inhibition is rapid, saturable at low-doses, that is, dose-dependent, irreversible and permanent for the entire life of the platelet - as it does not present with a biosynthetic mechanism to synthesize new proteins. After a single 325 mg dose of aspirin, the activity of platelet COX-1 recovers by approximately 10% per day due to new platelet formation. Aspirin also has dose-dependent antithrombotic effects on platelet function and blood coagulation that are not related to its ability to inhibit platelet COX-1 [12].
Aspirin can also influence hemostasis and cardiovascular diseases by the independent mechanisms of prostaglandin (PG) production [1,2]. Although not clearly defined, the effects of aspirin, which are not mediated by PG on hemostasis are postulated as being dose-dependent and not related to the activity of COX-1 [1,2]. These effects include vitamin K antagonism, reduction in the platelet production of thrombin and acetylation of one or more coagulation factors [13]. Aspirin can also harm platelet function by inhibiting platelet activation mediated by neutrophils [14]. Additionally, aspirin can potentially alter the pathogenesis of cardiovascular disease by protecting low-density lipoproteins from oxidative changes, on improving endothelial dysfunction in patients with atherosclerosis and attenuating inflammatory response by acting as an antioxidant [14].
The anti-inflammatory properties of aspirin are evident, but not well understood [1,2]. Treatment with aspirin inhibits endothelial dysfunction mediated by inflammatory processes, although the mechanism responsible for this effect is still unknown [15]. Moreover, in normal patients, therapy with low-doses of aspirin proved to reduce interleukin 7 released by platelets and thus to reduce the plasma levels of this cytokine [15]. These observations suggest that part of the beneficial effects of aspirin may be a reduction of vascular inflammation.
Pharmacology
The pharmacological composition of aspirin is characterized by a rapid absorption in the gastrointestinal tract with peak plasma concentrations between 30 and 40 minutes after intake [16]. The significant platelet inhibition effect is observed within 60 minutes of intake and one single 100 mg dose of aspirin completely blocks the production of TXA2 during the entire life of platelets in most individuals [16]. The plasma half-life of aspirin is close to 20 minutes, but the irreversible nature of COX-1 activity inhibition and the duration of TXA2 suppression demonstrate that the antithrombotic effects of aspirin are maintained with dose intervals of from 24 to 48 hours [16].
Randomized studies demonstrate that the benefits of aspirin therapy are achieved with a broad range of doses (from 30 to1500 mg per day), but the ideal daily dose has still not been clearly determined [6,7]. Generally, therapeutic regimes using high doses are not associated with significant additional benefits, but, may eventually attenuate the antithrombotic effects of aspirin and increase the risk of adverse effects [13].
Measurement of platelet function
To measure platelet function is difficult. In response to stimuli of platelet activation, they release several chemokines, cytokines and growth factors in preformed granules; synthesize prostanoids of arachidonic acid or transform messenger ribonucleic acids into proteins [1,2]. Apart from playing an important role in hemostasis, platelets also participate in inflammatory processes and in systemic responses to vascular injury [1,2]. In spite of the complex platelet function, laboratorial methods utilized to quantify the antithrombotic effects of aspirin are based on the measurement of platelet aggregation [1,2].
ASPIRIN RESISTANCE
Definition
The term "aspirin resistance" is currently utilized to describe numerous phenomena, including the inability of aspirin, to protect patients from thrombotic complications, to prolong the bleeding time and to inhibit the biosynthesis of platelet TXA2 [17]. The presence of recurrent vascular events in some patients however, in spite of prolonged aspirin therapy, should be denominated therapeutic failure instead of aspirin resistance, due to the multifactorial nature of atherothrombosis; only a small fraction of all the vascular complications can be prevented by one single preventive strategy [17].
Aspirin resistance, however, may be defined as either clinical or laboratorial resistance. Clinical resistance is defined as the failure of aspirin to prevent ischemic atherothrombotic events in patients with a high risk of developing cardiovascular and cerebrovascular diseases [18]. Laboratorial resistance, on the other hand, is the failure of aspirin to inhibit the production of platelet TXA2 or to inhibit the platelet function (platelet aggregation) that are dependent on platelet production of thromboxane [18].
Prevalence
The prevalence of laboratorial aspirin resistance varies from 5.5% to 61% [18]. Initial evidence that some patients are resistant to the antiplatelet action of aspirin are from the study by Mehta et al. [19] in which 30% of patients with coronary artery disease presented a minimum inhibition of platelet aggregation after one single 650 mg oral dose of aspirin. Buchanan et al. [7] already reported that bleeding time was prolonged in 23 of 40 patients hospitalized for elective coronary artery bypass grafting surgery, who took 325 mg per day of aspirin, suggesting a prevalence of aspirin resistance of 57.5%. Recently, Gabriel et al. [20], demonstrated that 12 of 41 heart disease patients who took daily aspirin doses of 100 mg as the only platelet antiaggregant drug, presented values of normal platelet aggregation or hyperaggregation, identifying aspirin resistance in 29% of these patients.
This variability of platelet inhibition mediated by aspirin was not demonstrated only in patients with coronary artery disease and in those submitted to coronary artery bypass grafting, but also in normal individuals and those with cerebrovascular diseases [21]. Despite of the apparent consistency of these observations, they are not reliable due to the small sample sizes, the different types of patients analyzed (variations in age, gender, ethnical background and clinical conditions), the different definitions of aspirin resistance, the uncertainty regarding the therapeutic response to the medication and the lack of compatibility between the different platelet function tests. The exact prevalence of aspirin resistance is still unknown.
Clinical relevance
The clinical observations suggest a causal relationship between aspirin resistance and cardiovascular risk [1,2]. One of the first works that demonstrated an association between the laboratorial measurement of aspirin resistance and the risk of severe vascular events was a cohort study of 181 stroke patients treated with high doses of aspirin (500 mg three times per day). In this research, an incidence of 30% of aspirin resistance was reported [22]. Grundmann et al. [23] found a significantly higher incidence of aspirin resistance (34%) among patients who had previously suffered from strokes or ischemic events, when compared to asymptomatic patients with cerebrovascular disease. Among patients with intermittent claudication submitted to peripheral vascular angioplasty, Mueller et al. [24] reported an incidence of 40% aspirin resistance with aspirin resistance being associated with an 87% increase in risk of future arterial occlusion after 18 months of clinical follow-up. In 2003, Gum et al. [9] reported on a cohort of 326 patients with cerebrovascular and coronary artery diseases, treated with 325 mg of aspirin per day who were clinically followed-up for two years. Of the 17 (5%) patients diagnosed with aspirin resistance, five (29%) suffered from serious vascular events in this period. Recently, Chen et al. [8] reported an association between aspirin resistance and creatine-kinase MB elevation after percutaneous coronary artery procedures.
In 151 patients with stable coronary artery disease, the incidence of aspirin resistance was 19.2%. Moreover, researchers of the PURSUIT study reported that previously taking aspirin is an independent predictor of increased cardiovascular risk among patients with acute coronary syndromes [25]. Of 9461 patients, those that had previously taken aspirin presented a 20% higher risk of suffering recurrent events in the following six months than those that had not utilized the medication [25].
Although widely utilized in primary and secondary prevention of cardiovascular events and in the management of patients submitted to coronary artery bypass grafting surgery, the ideal daily dose recommended for aspirin therapy has not been clearly elucidated yet. In 2001, the Brazilian Guidelines on Dyslipidemia and Prevention of Atherosclerosis [26] recommended doses of aspirin of greater than 100 mg per day for individuals who present with high risk for cardiovascular events (secondary prevention, diabetics or absolute risk of coronary events = 20% over 10 years) and those who do not present with contra-indications - degree of recommendation A and level of evidence 1. The guidelines in coronary artery bypass grafting, heart valve defect and aortic disease surgeries [27] recommend that patients submitted to coronary artery bypass grafting in acute coronary syndromes should take aspirin at doses of from 100 to 325 mg per day, starting in the first 24 postoperative hours and continuing indefinitely. In cases of aspirin intolerance, 250 mg per day of ticlopidine is recommended. On the other hand, guidelines for chronic coronary disease and stable angina [28], affirm that all individuals with stable angina must take aspirin, but the guidelines do not specify an ideal daily dose.
Despite the recommended dose of aspirin varying (from 30 to 1500 mg/day), with antiplatelet therapeutic indications for several clinical entities, such as heart arrhythmias, acute coronary syndromes, cerebrovascular diseases and peripheral vascular diseases, it is well known that for 5% to 45% of patients, aspirin does not produce antiplatelet effects and atherothrombotic events recur. Thus, further research is essential with the aim of establishing specific parameters that contribute to the indication of an effective dose for platelet antiaggregation. Moreover, the development of adequate techniques to measure the efficiency of aspirin as an antiplatelet agent will help to reduce recurrent vascular events following the use of aspirin.
Potential mechanisms of aspirin resistance
In spite of current knowledge related to the antiplatelet effects of aspirin, the mechanisms by which some patients are resistant are not clearly established yet [1,2]. The answer for this is probably a combination of genetical, biological and clinical aspects, that directly and indirectly affect platelet function (Table 1) [1,2]. From the clinical perspective, habits such as smoking, compliance with prescribed therapy, drug interactions for example with non-hormonal anti-inflammatory agents, and length of treatment with aspirin can contribute to individual differences regarding response to antiaggregation effects [1,2]. Although smoking has been identified by some researchers as a potent platelet activator and intensifier of thrombogenesis mediated by platelets, even with the suppression of TXA2 by aspirin, these observations are not consistent, showing the necessity of more research to study the influence of smoking on aspirin resistance [29]. Some researchers affirm that the increase in risk of recurrent vascular events in patients who take aspirin can be primarily explained by non-compliance to treatment. Cotter et al. [30] reported that among 73 patients who used aspirin on a daily basis after myocardial infarction, the percentage of adverse events such as sudden death, myocardial infarction or unstable angina over 12 months, was greater among patients who were considered non-compliers to treatment (42%) compared to those who were considered compliers (6%) or biologically resistant (11%). The role of non-hormonal anti-inflammatory drugs in the attenuation of the long-term antithrombotic benefits of aspirin has been reported, but this remains controversial [31]. Non-selective non-hormonal anti-inflammatory drugs have a strong affinity to a specific area of platelet COX-1 and may prevent aspirin mediated acetylation. However, recent studies are not consistent or definitive in providing clinical relevance to this potential interaction [1,2,31].
The length of therapy may also contribute to the lack of response to aspirin. Pulcinelli et al. [32] recently reported the effects of 2, 6, 12 and 24 months of aspirin treatment (100 or 330 mg per day) on ADP and platelet aggregation induced by collagen, among 150 patients with clinical evidence of atherothrombosis. In spite of adequate platelet inhibition after two months of treatment, over the long-term, treatment with aspirin was associated with a progressive reduction in sensitivity to its effects [32]. The researchers also affirmed that the sensitivity to aspirin is not dose-dependent, in agreement with previous observations.
The different mechanisms of platelet activation and the receptors involved may also contribute to the genesis of aspirin resistance. More specifically, mechanisms involving the non-dependent platelet activators of TXA2, such as thrombin, ADP, epinephrine and collagen, may prevent the aspirin mediated inhibitory effect leading to platelet activation and thrombosis. Platelet aggregation induced by catecholamines is, for example, one of the pathways that may not be adequately inhibited by aspirin [1,2].
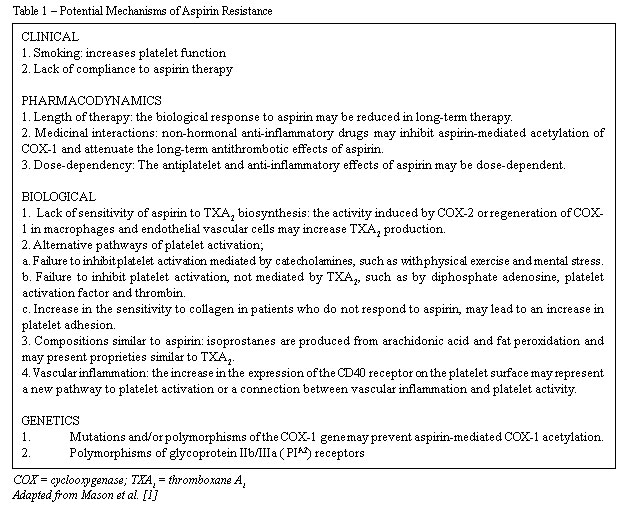
Hurlen et al. [33] reported that, among myocardial infarction patients, aspirin achieved satisfactory antiplatelet effects during resting, but failed to inhibit the increase of platelet aggregation induced by exercise. This observation suggests that the antiplatelet effects of aspirin can be surpassed over periods of increased activity of the sympathetic nervous system, such as during exercise or physical stress. Moreover, patients that do not respond to aspirin may present an increase of platelet sensitivity with collagen [34].
Alternative pathways of TXA2 synthesis and the identification of components similar to prostaglandin (isoprostane) also provide potential explanations of aspirin resistance syndrome. The biosynthesis of TXA2 that is not sensitive to aspirin may occur as a result of COX-2 induction in non-platelet cells (monocytes/macrophages or endothelial cells), resulting from a local inflammatory stimulus [35]. These cells can also release TXA2 or supply their respective precursor, PGH2, for platelet inhibition by aspirin. Moreover, COX-2 is present in newly-formed platelets and may contribute to the detectable levels of TXA2 synthesis during periods of platelet turnover [14]. Isoprostanes from fat peroxidation circulate in high concentrations in patients with unstable angina, diabetes mellitus and hyperlipidemia and in smokers. Moreover, acting as vasoconstrictors, isoprostanes may play a role in the amplification of platelet response to other agonists [13].
The interaction of the platelets with other cells, such as the red blood cells or vascular endothelial cells, may also affect aspirin-mediated inhibition. It has been demonstrated, for example, that red blood cells induce an increase in the platelet synthesis of TXA2 and release of serotonin, beta-thromboglobulin, and ADP. Previous investigations among patients with vascular diseases demonstrated that aspirin (200 to 300mg per day) incompletely blocks platelet reactivity in more than two-thirds of patients due to erythrocytes, in spite of an adequate inhibition of platelet TXA2 synthesis [17,18]. The increase in monocyte aggregation to circulating platelets during acute atherothrombotic events represents a link between inflammatory processes and thrombosis. There is evidence that interactions between CD40-CD40 receptors may play an important role in platelet activation, arterial thrombosis and in the pathways of platelet-mediated vascular inflammation [36].
Finally, aspirin resistance may also be explained, in part, by genetical differences in COX-1 genes or in the complex glycoprotein IIb/IIIa receptors. Polymorphisms of the IIIa subunit have been identified and specific alleles, PlA1/A2 and PlA2/A2, are associated with an increase in thrombin formation and there is a low threshold of platelet activation, alpha granule release and fibrinogen binding [35]. Although not confirmed yet, it has been suggested that mutations or polymorphisms of COX-1 genes may also help to explain the structural basis of aspirin resistance in some patients [35].
Treatment of aspirin resistance
The best manner to treat aspiring resistance and, thus, to improve the efficacy of aspirin in preventing atherothrombotic vascular events, consists in identifying and treating the aspect responsible for aspirin resistance [18].
Adequate therapeutic alternatives include the identification and treatment of non-atherothrombotic causes of vascular events, that will probably not respond to aspirin, such as for example, antibiotics for infectious endocarditis and corticoids for arteritis; improvement in the therapeutic compliance of patients to aspirin treatment; avoid the use of medications that may adversely interact with aspirin, such as ibuprofen; cease smoking; increase the frequency of aspirin administration and substitute or associate aspirin with other antiplatelet agents capable of inhibiting other pathways responsible for platelet activation, such as blocking ADP receptors, thromboxane receptor antagonists or the common final pathway of platelet aggregation, such as blocking intravenous glycoprotein IIb/IIIa receptors [17,18].
However, although these therapeutic alternatives seem sufficient, they are not necessarily effective and safe. For example, an increase in the aspirin dose in order to increase platelet COX-1 suppression seems logical, but evidence shows that low doses of aspirin are as efficient as high doses in the prevention of cardiovascular events [18]. Moreover, randomized studies state that the addition of clopidogrel to aspirin therapy, in patients with acute coronary syndromes or submitted to percutaneous coronary interventions, improves the results without increased danger [37].
Thus, further research is necessary to determine the effectiveness and safety of other alternative therapies for aspirin resistance, as well as to identify factors that are associated to favorable or unfavorable responses.
CONCLUSION
Aspirin resistance remains a highly prevalent phenomenon in patients who require antithrombotic treatment and represents a biological aspect with significant clinical implications. It is important to mention that laboratorial measurement showing lack of antiaggregation effects to aspirin is a predictor of increased risk for future atherothrombotic events.
The main doubts that still need to be studied further in relation to aspirin are:
1. The lack of a standardized definition and a valid method to identify aspirin resistance;
2. The unknown prevalence of aspirin resistance in the population;
3. To identify clear biological mechanisms of aspirin resistance;
4. The incomplete understanding of adequate mechanisms in the action of aspirin related to the daily dose, variations in its effect with time, its interaction with other medications and hormones and genetical markers related to its activity;
5. The absence of a proven therapeutic strategy for affected individuals.
In spite of resistance to the antithrombotic effects of aspirin, this medicine continues to participate as a powerful therapy against atherothrombotic complications in cardiovascular diseases.
REFERENCES
1. Mason PJ, Jacobs AK, Freedman JE. Aspirin resistance and atherothrombotic disease. J Am Coll Cardiol. 2005;46(6):986-93.
2. Mckee SA, Sane DC, Deliargyris EN. Aspirin resistance in cardiovascular disease: a review of prevalence, mechanisms, and clinical significance. Thromb Haemost. 2002;88(5):711-5.
3. Ribeiro RA, Mello RG, Melchior R, Dill JC, Hohmann CB, Lucchese AM, et al. Custo anual do manejo da cardiopatia isquêmica crônica no Brasil: perspectiva pública e privada. Arq Bras Cardiol. 2005;85(1):3-8.
4. Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy. I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308(6921):81-106.
5. Steering Committee of the Physicians' Health Study Research Group. Final report on the aspirin component of the ongoing Physicians' Health study. N Engl J Med. 1989;321(3):129-35.
6. Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BJM. 2002;324:71-86.
7. Buchanan MR, Brister SJ. Individual variation in the effects of ASA on platelet function: implications for the use of ASA clinically. Can J Cardiol. 1995;11(3):221-7.
8. Chen WH, Lee PY, Ng W, Tse HF, Lau CP. Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J Am Coll Cardiol. 2004;43(6):1122-6.
9. Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003;41(6):961-5.
10. McNicol A, Israels SJ. Platelets and anti-platelet therapy. J Pharmacol Sci. 2003;93(4):381-96.
11. Patrono C, Bachmann F, Baigent C, Bode C, De Caterina R, Charbonnier B, et al. Expert consensus document on the use of antiplatelet agents. The Task Force on the Use of Antiplatelet Agents in Patients with Atherosclerotic Cardiovascular Disease of the European Society of Cardiology. Eur Heart J. 2004;25(2):166-81.
12. Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):234S-64S.
13. Patrono C, Coller B, Dalen JE, FitzGerald GA, Fuster V, Gent M, et al. Platelet-active drugs: the relationships among dose, effectiveness, and side effects. Chest. 2001;119(1 suppl.):39S-63S.
14. Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101(10):1206-18.
15. Damas JK, Waehre T, Yndestad A, Otterdal K, Hognestad A, Solum NO, et al. Interleukin-7-mediated inflammation in unstable angina: possible role of chemokines and platelets. Circulation. 2003;107(21):2670-6.
16. Patrono C. Aspirin as an antiplatelet drug. N Engl J Med. 1994;330(18):1287-94.
17. Patrono C. Aspirin resistance: definition, mechanisms and clinical read-outs. J Thromb Haemost. 2003;1(18):1710-3.
18. Hankey GJ, Eikelboom JW. Aspirin resistance. Lancet. 2006;367(9510):606-17.
19. Mehta J, Mehta P, Burger C, Pepine CJ. Platelet aggregation studies in coronary artery disease. Atherosclerosis. 1978;31(2):169-75.
20. Gabriel SA, Tristão CK, Izar LC, Domingues C, Gabriel EA, Cliquet MG. Avaliação da agregação plaquetária e dosagem do fibrinogênio em pacientes com doenças cardiovasculares e sua correlação com o uso de aspirina e fatores de risco coronariano. Rev Bras Cir Cardiovasc. 2006;21(3):289-94.
21. Pappas JM, Westengard JC, Bull BS. Population variability in the effect of aspirin on platelet function. Implications for clinical trials and therapy. Arch Pathol Lab Med. 1994;118(8):801-4.
22. Grotemeyer KH, Scharafinski HW, Husstedt IW. Two-year follow-up of aspirin responder and aspirin non-responders. A pilot-study including 180 post-stroke patients. Thromb Res. 1993;71(5):397-403.
23. Grundmann K, Jaschonek K, Kleine B, Dichgans J, Topka H. Aspirin non-responder status in patients with recurrent cerebral ischemic attacks. J Neurol. 2003;250(1):63-6.
24. Mueller MR, Salat A, Stangl P, Murabito M, Pulaki S, Boehm D, et al. Variable platelet response to low-dose ASA and the risk of limb deterioration in patients submitted to peripheral arterial angioplasty. Thromb Haemost. 1997;78(3):1003-7.
25. Alexander JH, Harrington RA, Tuttle RH, Berdan LG, Lincoff AM, Deckers, JW, et al. Prior aspirin use predicts worse outcomes in patients with non-ST-elevation acute coronary syndromes. PURSUIT Investigators. Platelet IIb/IIIa in Unstable angina: Receptor Suppression Using Integrilin Therapy. Am J Cardiol. 1999;83(8):1147-51.
26. III Diretrizes Brasileiras sobre Dislipidemias e Diretriz de Prevenção da Aterosclerose do Departamento de Aterosclerose da Sociedade Brasileira de Cardiologia. Arq Bras Cardiol. 2001;77(supl 3):1-48.
27. Lima RC, Kubrusly LF, Nery AC, Pinheiro BB, Brick AV, Souza DSR, et al. Diretrizes da cirurgia de revascularização miocárdica, valvopatias e doenças da aorta. Arq Bras Cardiol. 2004;82(suppl 5):1-20.
28. César LAM, Mansur AP, Armaganijan D, Amino JG, Sousa AC, Simão AF, et al. Diretrizes de doença coronariana crônica angina estável. Arq Bras Cardiol. 2004;83(suppl 2): 2-43.
29. Brockmann MA, Beythien C, Magens MM, Wilckens V, Kuehnl P, Gutensohn K. Platelet hemostasis capacity in smokers. In vitro function analyses with 3.2% citrated whole blood. Thromb Res. 2001;104(5):333-42.
30. Cotter G, Shemesh E, Zehavi M, Dinur I, Rudnick A, Milo O, et al. Lack of aspirin effect: aspirin resistance or resistance to taking aspirin? Am Heart J. 2004;147(2):293-300.
31. Kurth T, Glynn RJ, Walker AM, Chan KA, Buring JE, Hennekens CH, et al. Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal antiinflammatory drugs. Circulation. 2003;108(10):1191-5.
32. Pulcinelli FM, Pignatelli P, Celestini A, Riondino S, Gazzaniga PP, Violi F. Inhibition of platelet aggregation by aspirin progressively decreases in long-term treated patients. J Am Coll Cardiol. 2004;43(6):979-84.
33. Hurlen M, Seljeflot I, Arnesen H. Increased platelet aggregability during exercise in patients with previous myocardial infarction. Lack of inhibition by aspirin. Thromb Res. 2000;99(5):487-94.
34. Kawasaki T, Ozeki Y, Igawa T, Kambayahsi J. Increased platelet sensitivity to collagen in individuals resistant to low-dose aspirin. Stroke. 2000;31(3):591-5.
35. Cipollone F, Rocca B, Patrono C. Ciclooxygenase-2 expression and inhibition in atherothrombosis. Arterioscler Thromb Vasc Biol. 2004;24(2):246-55.
36. Freedman JE. CD40-CD40L and platelet function: beyond hemostasis. Cir Res. 2003;92(9):944-6.
37. Sabatine MS, Cannon CP, Gibson CM, Lopez-Sendon JL, Montalescot G, Theroux P, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352(25):1179-89.

 All scientific articles published at bjcvs.org are licensed under a Creative Commons license
All scientific articles published at bjcvs.org are licensed under a Creative Commons license