To evaluate the Lecompte procedure used for the correction of the transposition of the great arteries (TGA) associated with ventricular septal defect (VSD) and the left ventricle outflow tract obstruction (LVOTO) and to present the intermediate and long-term results of the surgery.
Between February 1994 and July 2005, seven patients with ages between 2 and 8 years (median: 3.0) suffering from TGA, VSD and LVOTO underwent corrective surgery. In six cases, the Lecompte procedure was performed. This technique consists in right ventriculotomy, extensive resection of conal septum and construction of a ventricular tunnel connecting the left ventricle to the aorta (LV-Ao). The remaining case presented with obstruction of a valvular prosthesis implanted between the right ventricle and the pulmonary artery (RV-PA) and RV failure. This case was converted to the Lecompte procedure.
The cardiopulmonary bypass time varied from 105 to 194 minutes (Median: 130) and the aortic clamping time varied from 65 to 90 minutes (Median: 78). There was one death in the immediate post-operative period due to coagulopathy followed by RV failure. Six patients were released from hospital between the 5th to 30th postoperative day (Median: 11) and the follow up period was from 12 to 144 months (Median: 73.6).
The Lecompte procedure presents the following advantages: 1. Surgical indicationfor infants; 2. Low morbility and mortality rates; 3.Free from reoperation over the long term. 4. Possibility of conversion of the Rastelli procedure into the Lecompte procedure.
Avaliar o procedimento de Lecompte para a correção da transposição das grandes artérias associada à comunicação interventricular e obstrução da via de saída do ventrículo esquerdo (TGA, CIV e OVSVE) e apresentar os resultados no período pós-operatório intermediário e tardio.
Entre fevereiro de 1994 e julho de 2005, sete pacientes, com idade de 2 a 8 anos (mediana -M-: 3,0), portadores de TGA, CIV e OVSVE, foram submetidos a tratamento cirúrgico corretivo. Em seis casos, foi utilizado o procedimento de Lecompte. Esta técnica consiste na abordagem por ventriculotomia direita, ressecção ampla do septo conal e construção de um túnel ventricular conectando o ventrículo esquerdo à aorta; o caso restante apresentava obstrução da prótese valvulada implantada entre o ventrículo direito e a artéria pulmonar (VD-AP) e falência do VD e foi submetido à conversão no procedimento de Lecompte.
Os tempos de CEC variaram entre 105 e 194 min (M: 130) e os tempos de anoxia entre 65 e 90 min (M: 78). Houve um óbito no pós-operatório imediato devido a coagulopatia, seguido de insuficiência ventricular direita. Os seis pacientes sobreviventes receberam alta hospitalar no período de 5 a 30 dias (M: 11) e permaneceram em acompanhamento entre 12 a 144 meses (M: 73,6).
O procedimento de Lecompte teve como vantagens: 1 - Indicação cirúrgica em pacientes com menor faixa etária; 2 - Baixa morbi-mortalidade; 3 - Expectativa de acompanhamento a longo prazo, sem reoperação; 4 - Possibilidade de converter o procedimento de Rastelli em Lecompte.
INTRODUCTION
For transposition of the great arteries (TGA), anatomic correction has been the procedure of choice, giving good results in the immediate and late postoperative periods. When TGA is associated to interventricular communication (IVC) and obstruction of the left ventricle outflow tract (LVOFT), the complexity of a surgical correction increases with this option becoming less attractive.
In the last three decades, the operation described by Gian Carlo Rastelli et al. [1], in 1969, has been considered the first-line procedure for the surgical correction of TGA, IVC and LVOFT.
In the original description of Rastelli, after making a tunnel between the left ventricle (LV) and aorta (Ao), a connection is made between the right ventricle (RV) and pulmonary artery (PA) by means of the implantation of an extracardiac tube. The long-term evolution of these patients shows a growing incidence of adverse events, showing that the Rastelli operation, although being a good technical option for the correction of TGA, IVC and LVOFT, is not the ideal procedure when: 1 - the intracardiac autonomy is not favorable; 2 - it is necessary to indicate the operation in younger patients (the extracardiac prosthesis in under two year-old patients, rapidly evolves to occlusion) and 3 - there is an option for other surgical procedures with less incidence of obstruction of the right ventricle outflow tract (RVOFT) or LVOFT.
In view of the growing number of reoperations in the long-term evolution of the Rastelli operation, we suggest other technical alternatives that avoid the use of extracardiac tubes.
Yves Lecompte, et al. [2] in 1982 presented the "Réparation a l'etage ventriculair" a new alternative for the surgical correction of TGA, IVC and LVOFT, introducing technical changes in the preparation of the LV-Ao tunnel and innovating concepts in the reconstruction of the RVOFT without the use of extracardiac prostheses.
Other advantages with the Lecompte procedure are the technical resources available to the surgeon faced with anatomic variations which may be present such as: restrictive IVC (must be enlarged), anomalous implantation of the tricuspid valve (the papillary muscle may be sectioned and re-implanted) the presence of obstructive conal septum (must be resected).
On the other hand, there are contraindications of anatomic order, such as: IVC distant from the aortic valve or multiple IVC, making the preparation of the LV-Ao tunnel more difficult: hyperplasia of the RV or LV and diffuse hyperplasia of the pulmonary arteries.
Since 1994, the Lecompte operation is being routinely used in the cardiovascular surgery department of the Federal University of Sao Paulo (UNIFESP).
Over the last 12 years, seven patients with TGA, IDC and LVOFT were operated on using the Lecompte technique. In this work, the technical aspects of the operation and results of immediate and long-term postoperative evolution will be analysed.
METHOD
Patients
Between February 1994 and July 2005 seven patients with TGA, IVC and LVOFT who were submitted to surgical correction using the Lecompte procedure were analysed. Six cases were referred electively and one case (already operated) as an urgency. The ages of the patients at operation ranged between 2 and 8 years old (median 3.0). Five patients were less than five years of age and four were less than three years old (Table 1).
The clinical diagnoses were confirmed for all patients using Doppler echocardiography and hemodynamic and cineangiographic studies. Three patients presented with restrictive IVC and two patients were submitted to atrioseptostomy (Rashkind) in the first year of life.
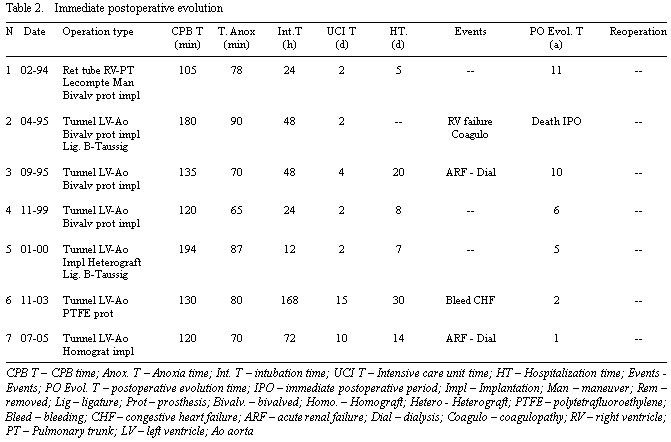
Five patients presented with the pulmonary trunk (PT) positioned posteriorly to the Ao and in two cases the PT was to the right of the Ao. The valve and ring of the PT, in all cases, presented hyperplastic with adequate pulmonary blood flow in four cases (patients 3, 4, 6 and 7) and low in the other three cases which had been the reason for previous operations: Patient Nº 1 was operated on using the Rastelli technique at for years of age, in a third postoperative year the infant presented with obstruction of the extracardiac valved prosthesis, located to the right of the Ao between the RV and the PA, which evolved with severe dysfunction of the RV followed by low heart output. The other two patients (Patients 2 and 5) were submitted to the modified Blalock-Taussig operation with polytetrafluoroethylene (PTFE) prostheses, within the first six months of life (Table 2).
Operative technique
All the patients were operated on using median sternotomy. On opening the pericardium, an inspection of the cardiac anatomy was made, focusing on the position of the great vessels in relation to the heart chambers (Figure 1).
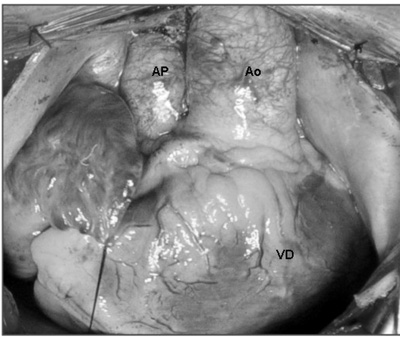
Fig. 1 - Surgery photograph - observe the heart structures after opening the pericardium. Aorta (Ao) in the anterior position, originating from the right ventricle (RV) and the pulmonary artery (PA) in the posterior position to the right of the Ao
Preparation prior to the establishment of cardiopulmonary bypass (CPB) consisted in dissection of the ascending Ao (along its complete length), the great vessels and both pulmonary arteries to their bifurcation near to the pericardium. At the same time, the modified Blalock-Taussig procedure was dissected and using ligatures it was sectioned.
In all cases CPB was utilized with moderate hypothermia; the arterial flow was achieved using cannulization of the ascending Ao near to the brachiocephalic trunk and venous drainage using cannulae positioned in both vena cavas. The left chambers were emptied by placing a 'vent' catheter in the LV introduced through the right upper pulmonary vein.
Myocardial protection, after aortic clamping, was achieved with the administration of sanguineous cardioplegia solution injected anterogradely (root of the Ao) at 20 minute intervals. At the same time, the pericardial cavity was irrigated with cold saline solution (surface hypothermia).
To construct the tunnel between the LV-Ao, the approach was by an oblique minimal ventriculotomy in the RV infundibulum, below the aortic ring, far from the main and secondary branches of the coronary arteries, facilitating access to the structures of the right ventricle cavity.
The basic principle of this operation is to keep the IVC aligned with the Ao to make the tunnel, preventing intra-tunnel gradients. For this, ample resection of the conal septum is normally necessary and was performed in six patients in our series. The IVC was assessed in relation to its location and diameter, comparing it with the diameter of the aortic ring. When these diameters were not proportional, the IVC is considered restrictive and enlarged at its anterior face. Three patients of our series had the IVC enlarged (Figure 2).

Fig. 2 - Surgery photograph - Patient under perfusion with the cannulae in the aorta (Ao) and vena cavas, cannulated by the right atrium (RA). Approach made by the right ventricle (RV) using right ventriculotomy (right ventricult.) at the infundibulum (Infund.) - observe the restrictive intraventricular connection (IVC) Anterior enlargement of the IVC was performed (Ant. Amp. IVC)
The LV-Ao tunnel was prepared with an oval patch of biological (bovine pericardium) or synthetic (PTFE) tissue, utilizing additional sutures at the edge of the IVC, aortic ring and ring of the tricuspid valve (Figure 3).

Fig. 3 - Surgery photograph - Aspect of the anterior face of the right ventricle (RV). Approach of heart defects by right ventriculotomy to make a tunnel between the left ventricle and the aorta (LV-Ao tunnel) with a polytetrafluoroethylene (PTFE) patch
Anomalous insertion of the tricuspid valve was found in none of the cases in our series.
For the reconstruction of the RVOFT, dissection of the pulmonary arteries up to the origin of their branches and release of the mediastinum structures were necessary. Subsequently, the Ao and the PT were sectioned directly above the valves and the proximal neck of he PT was closed.
In our series, closure of the proximal neck of the PT using double suture lines was eventually adopted (Figure 4).
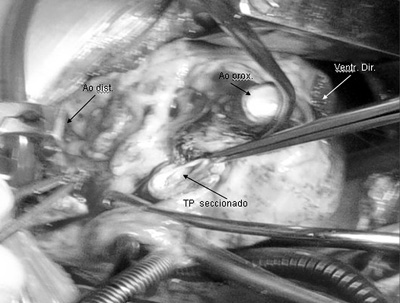
Fig. 4 - Surgery photograph - Observe the crosswise sectioniung of the aorta (Ao) showing its proximal (prox Ao) and distal (distal Ao) necks. The hypoplastic pulmonary trunk (PT) is being sectioned above its valve. Right ventriculotomy (Right ventr.)
Following this, the PA is placed forward of the Ao (Lecompte maneuver), the ascending Ao is shortened, when necessary, by crosswise sectioning of a cylinder 1.5 to 2.0 cm in width and the Ao is reconstructed with an end-to-end anastomosis using a continuous suture line.
In all patients in our series, the Lecompte maneuver was performed and in four it was necessary to shorten the ascending Ao (Figure 5).
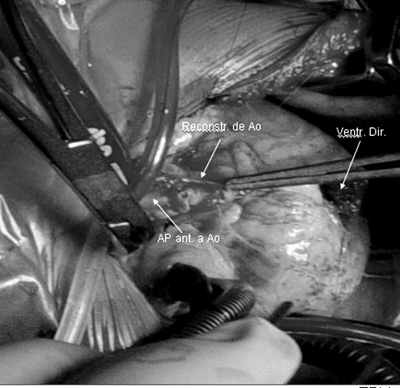
Fig. 5 - Surgery photograph - Observe the PA brought forward in respect to the Ao (Lecompte maneuver) and the Ao being reconstructed using an end-to-end anastomosis. Right ventriculotomy (Right ventr.)
After closure of the IAC, prior to removal of the air from the left cavities and with the heart beating after declamping the Ao, reconstruction of the RV-PT junction was performed.
The posterior portion of the PT was sutured at an angle above the ventriculotomy and, on its anterior face porcine pulmonary bivalved prostheses were implanted in four patients (Figure 6) and a pulmonary homograft in one case. These prostheses were adjusted at the RV-PT junction to prevent residual pulmonary valve insufficiency (PVI). The site of the anastomosis of the PT in the RV was based on the anatomical characteristics of each case: when the Ao and the PT were in anteroposterior position, it is possible to bring them together and perform the right connection between the RV and the PT; when the PT is to the right of the Ao, bringing them together is more difficult with the necessity of the use of a tubular prosthesis associated with the Lecompte procedure or not. Two of the patients in our series presented with the PT to the right of the Ao with the necessity of reconstruction of the RVOFT using PTFE prosthesis and a porcine pulmonary heterograft (Figure 7).
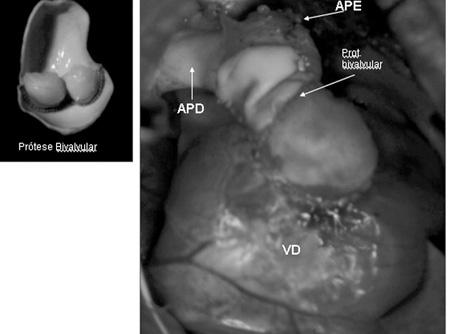
Fig. 6 - Surgery photograph - Reconstruction of the right ventricle junction (RV) - pulmonary artery (PA) with porcine pulmonary bivalved prosthesis (Bivalved prosth.)

Fig. 7 - Surgery photograph - Reconstruction of the right ventricle junction (RV) - pulmonary artery (PA) with pulmonary heterograft (P Hetero.)
It was also necessary to find an adequate site in the mediastinum to place these prostheses to prevent their compression during the closure of the chest.
The first patient of this series, submitted to the Rastelli operation in another hospital three years previously, presented with acute obstruction of the valved tube, placed between the RV and the PA. Acute failure of the RV obliged us to perform an emergency operation. A woven-Dacron tube with a biological prosthesis was found positioned in front of and to the right of the Ao; at its emergence from the RV, it presented with signs of compression by the sternum, a probable origin of thrombosis. The prosthesis was carefully resected, the epicardiac adherences released and it was removed in one piece, avoiding lesions to the coronary arteries.
To reconstruct the RVOFT without tension, ample release of PA adherences to the mediastinum was necessary and shortening of the Ao, by sectioning a 2.0-cm wide cylinder, thereby allowing a better approach of the PT to the RV infundibulum. Finally, a bivalved porcine prosthesis was utilized for the reconstruction of the pulmonary valve (Figure 6).
In all cases, conventional ultrafiltration was utilized during the warming phase of perfusion and modified ultrafiltration after completing CPB, maintaining the venous cannula in the right atrium and arterial in the ascending Ao. The use of ultrafiltration allowed the normalization fluid volume, a reduction of edema of the tissue and in the size of the heart chambers, enabling closure of the chest in all cases without risk of compression of the RVOFT.
RESULTS
The surgical procedure was performed with aortic clamping (myocardial anoxia) which varied between 65 and 90 minutes (median = 78 minutes) and a CPB time which ranged between 105 and 194 minutes (median = 130 minutes) - Table 2.
Immediately after CPB, the pressures of the heart chambers were measured and no LV/RV pressure relationship greater than 50% was observed in any of the cases.
There was one death in this series (Patient nº 2) on the second postoperative day giving a mortality rate of 14.2%. This patient presented with significant post-CPB bleeding due to a leak of the suture at the proximal neck of the PT with the necessity of reestablishing CPB due to difficulties to access the bleeding site (posterior face of the Ao). The bleeding was controlled, however the patient evolved with a coagulopathy followed by right ventricular failure.
The times of ventilatory support ranged from 12 hours to 168 hours (median = 48 hours). One patient presented with acute respiratory insufficiency (ARI) with a prolonged pulmonary ventilation time (168 hours) due to congestive heart failure (CHF), caused by residual pulmonary valve failure. The other patients were extubated in 12 to 70 hours.
The surviving six patients remained in the pediatric ICU between 2 and 15 days (median = 2). Two patients (Patients nº 3 and 7) presented with oliguria which was reverted by peritoneal dialysis for 24 to 48 hours.
One patient presented with diffuse bleeding of the thoracic wall with the necessity of reoperation to remove blood clots from the mediastinum.
The surviving patients were released from hospital between the 5th and 30th postoperative days (median = 11 days).
The postoperative follow up varied from 12 to 144 months (median = 73.6 months). All the patients had sinus rhythm. Five patients are in Functional Class I (NYHA) and one patient in Functional Class II using specific medication due to moderate PVI (RV-PA PTFE tube).
At chest radiography, no patient presented with signs of calcification of the pulmonary prostheses.
Serial Doppler echocardiography performed six months after the operation did not show significant pressure gradients at the LV-Ao tunnel, although 3 patients presented with restrictive IVC submitted to enlargement. Only one patient (nº 6 with PTFE prosthesis) presented a gradient above 25 mmHg at the pulmonary prosthesis and moderate PVI; 3 patients (nº 1, 2 and 6) had PVI with slight to moderate hemodynamic repercussions but with the RV function preserved.
Two patients were submitted to control heart catheterisms. Patient nº 1 presented with the absence of a significant LV-Ao pressure gradient, moderate PVI and a RV-PA gradient of 12 mmHg (bivalved prosthesis); similarly Patient nº 7 did not present with a significant gradient between the LV-Ao and a RV-PA gradient of 16 mmHg and absence of regurgitation through the pulmonary valve (pulmonary homograft) - Figure 8.

Fig. 8 - Postoperative hemodynamic study - Right ventriculotomy with contrast of the pulmonary homograft (P homo) and the right pulmonary artery (RPA) and left pulmonary artery (LPA)
The survival in this group of patients was 85.8%. The follow up of the six surviving patients ranged from 12 to 144 months (median = 73.6 months) - Figure 9.
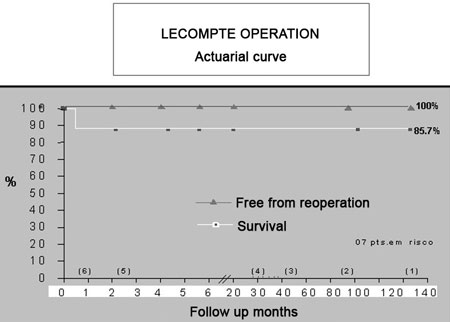
Fig. 9 - Actuarial curve showing the survival of the operated patients (85.7%) and the patients free from reoperations (100%) in a period of 12 to 144 months (median = 73.6 years)
Up to now, no patient was reoperated for the replacement of the pulmonary prosthesis in the medium and late postoperative periods.
DISCUSSION
The Rastelli operation [1] was one of the historic marks of heart surgery, creating a new repair technique for the combination of TGA-IVC-LVOFT based on redirecting the blood flow from the ventricle. This technique was considered the best option at that time however it is not the ideal solution.
In many centers, the Rastelli procedure requires an extracardiac tube for the reconstruction of the RVOFT, with some limitations in its use: 1 - This operation is not recommended in newborn or breastfeeding babies, as the use of a prosthesis proportional to the size of the patients structures becomes obstructive in a short period of time; 2 - The necessity of replacing the prosthesis in the mid-term, due to its progressive degenerative process including calcification, thrombosis, infection, a source of embolization, etc. [6]; 3 - the disproportion between the diameter of the prosthesis and the space for its accommodation in the mediastinum, causes obstruction of the prosthesis due to compression of the sternum [7]; myocardial ischemia by compression of the ring of the valve prosthesis on the coronary artery that crosses the anterior face of the heart, etc. [8].
The Lecompte procedure offers some advantages compared to the Rastelli operation such as: 1 - the possibility of restoring the anatomic continuity between the RV and the pulmonary arteries without the utilization of a tubular prosthesis; 2 - surgical indication in younger patients; 3 - possibility of anatomic conversion of many patients with non-satisfactory evolution using the Rastelli procedure into the Lecompte operation.
This operation also counts on other advantages such as 4 - the resection of the conal septum allowing a reduction of the LV-Ao intra-tunnel gradients; 5 - Potential for growth of the RV-PT junction, accompanying the development of he patient and reducing the incidence of reoperations.
The presentation of this group of patients has as its main objective to show the advantages of the Lecompte operation and the evolution of the patients taking into account their ages. The 2-year-old patients (Patients 3, 4 and 7) presented with good physical development free of reoperations at 7, 6 and 1 postoperative years, respectively.
Morbimortality in the immediate postoperative period
The Lecompte operation may be safely performed with low mortality rates and good long-term results.
The results of the surgical treatment of TGA, IVC and LVOFT with the available surgical procedures, have improved immensely over the last 15 years [6]. The mortality rate ranges from 3% to 18% [6,9], and is mainly related to low heart output syndrome due to the residual gradient between the LV-Ao, LV or RV hypoplasia, hypoplasia of the pulmonary arteries, lesion of the coronary arteries and residual mitral valve insufficiency.
In our series there was only one death (14.2% - Patient nº 2), due to a coagulopathy and RV failure on the 2nd postoperative day. The prolonged time of CPB, to correct bleeding at the suture of the proximal neck of the PT contributed to this fatal event.
With a change in the surgical technique, this event was not observed in the rest of the patients. These changes included: 1 - The crosswise sectioning of the PT was made farther from the pulmonary ring; 2 - The thickened or hypoplastic pulmonary valve was closed by sutures at the free edge of its valves; 3 - The edges of the wall of he PT proximal neck were closed with two suture lines, the first line with "U-shaped" stitches anchored on bovine pericardium strips and the other line using a continuous suture.
Obstruction of the right ventricle outflow tract
The obstruction of the extracardiac prosthesis in the Rastelli operation is a common occurrence in the mid to long-terms [6,10,11]. The decision to reoperate is influenced by the symptoms of the patient, the RV-PA pressure gradients and dysfunction of the RV. Tricuspid insufficiency shows the degree of obstruction of the prosthesis and indicates early reoperations [12,13]. Other studies show that patients with diverse degrees of obstruction of the prosthesis may be asymptomatic [10]. These prostheses should be substituted immediately after RV dysfunction begins.
Patients submitted to the Rastelli operation evolving with extracardiac prosthesis obstruction and with indication of reoperation, may be submitted to the conversion of the Rastelli operation to the Lecompte procedure [14].
In our experience, the first patient of the series who was operated on in another institution three years previously using the Rastelli operation, presented with acute obstruction of the valved prosthesis between the RV and the PA, followed by failure of the RV. After a hemodynamic study to confirm the diagnosis, the patient was submitted to an emergency operation. The valved tube was located to the right of the Ao and its emergence from the RV presented with signs of compression caused by the sternum probably an origin of thrombosis.
With a complete but careful resection of the prosthesis ("Woven-Dacron" tube with biological prosthesis) and shortening of the ascending Ao, a space was created in the mediastinum enabling reconstruction of the RV-PA function.
The Lecompte maneuver (forward placement of the PA) made reconstruction of the RVOFT easier without tension when the vessels (PT and Ao) were in an anteroposterior position (Patients nº 1, 2, 3, 4 and 7). When the vessels were side-by-side, the Lecompte maneuver is not recommended due to the difficulty to bring the PA and the RV together. A study performed by Lecompte et al. [15] of 50 patients showed that in 44 cases it was possible to use the Lecompte maneuver as the vessels were in the anteroposterior position and not side-by-side.
With Patients nº 5 and 6 of our series (PT to the right of the Ao), it was necessary to implant PTFE prostheses and biological valve grafts (heterografts) for the reconstruction of the RVOFT due to the impossibility of bringing the PT close to the infundibulum of the RV. In both cases the Lecompte maneuver was performed which improved the position of these structures utilizing shorter prostheses to the right of the Ao.
Reconstruction of the pulmonary valve
One of the most important considerations in the evaluation of the Lecompte procedure is the form in which the pulmonary valve will be reconstructed. Lecompte et al. [2] stressed in their publication that the PVI should be avoided by means of the implantation of some type of valved mechanism in the RV-PA connection. The implantation of a valved prosthesis prevents acute hemodynamic alterations, such as an increase in the RV final diastolic volume due to pulmonary regurgitation, allowing a better performance of the RV in the immediate postoperative period.
Him et al. [16] showed the possibility of bringing the PT close to the RV infundibulum on the right side of the Ao without performing the Lecompte maneuver even with the vessels in an anteroposterior position. To achieve an anastomosis between the PA and RV without tension the authors reported the necessity of ample dissection of the pulmonary arteries.
Metras et al. [17] developed a technique of auto-grafting to reconstruct the RV-PA junction. After crosswise sectioning of the ascending Ao, an asymmetric cylinder of between 16 and 20 mm of width of the vessel was removed. The PA was sectioned above the valve, its proximal neck was sutured, the Ao was reconstructed without performing the Lecompte maneuver and this cylinder of the Ao was placed between the RV and the PA to the right or left of the Ao leaving a residual PVI.
Monocuspid prostheses were employed more frequently to reduce the degree of pulmonary regurgitation. When implanted using a good technique, the degree of pulmonary insufficiency is slight, although its function is lost causing different degrees of PVI over the short term [6,17].
In spite of the excellent immediate results using monocuspid prostheses, progressive obstructions have been observed at the RV-AP junction due to the development of tissue calcification.
Obstruction of the RVOFT was reported by Vouhé et al. [14] in 26% of cases over a follow up of 55 months and Lee et al. [6] registered an incidence of 25%. All the patients were reoperated.
Not satisfied with the results, other types of prostheses were tested aiming at improving the performance and reducing the incidence of calcification including: 1 - development of monocuspids using other types of synthetic or biological tissues [6,18,19]; 2 - Auto-graft of the Ao, questioning the necessity of a valve at the RV-PA junction [17]; 3 - preservation of the pulmonary valve [20] and 4 - implantation of a homograft [21].
From the accumulated experience, the pulmonary homograft calls the attention because it has an expected long evolution free from calcification, a better performance and better survival free from reoperation for patients.
In our experience, four patients were submitted to reconstruction of the RV-PA with porcine pulmonary bivalved prostheses, preserving and taking advantage of the experience accumulated in the reconstruction of the RVOFT in tetralogy of Fallot [22]. During the follow-up of these patients, in the immediate and mid-term postoperative periods, there was a good adaptation and performance of these prostheses presenting with slight residual pulmonary insufficiency.
Two patients, one 11 years old and the other 12 years old, presented with moderate pulmonary insufficiency but without involvement of the RV function. One patient received a heterologous pulmonary prosthesis showing good evolution in the 5th postoperative year and one patient received a pulmonary homograft with good evolution in the first postoperative year. Both the patients presented with good valve function as identified by Doppler echocardiography. The second patient also underwent hemodynamic and cineangiographic examinations that confirmed this result. One patient received the implantation of a PTFE tubular prosthesis because it was impossible to bring the PT and the RV close together. The patient presented with moderate pulmonary insufficiency in the 2nd postoperative year although without demonstrating signs of dysfunction and with low RV pressures maintained.
Obstruction of the left ventricle outflow tract
When using the Lecompte procedure, the most important maneuver is the resection of the conal septum, leaving the IVC aligned with the aortic valve and allowing the preparation of the LV-Ao tunnel with a lower incidence of intra-tunnel gradients.
The IVC is considerable restrictive when its diameter is less than the diameter of the aortic valve and in these cases enlargement of the IVC at its anterior edge is necessary. In spite of all these precautions, significant LV-Ao gradients have been reported (> 30 mmHg) [6].
In our series, all the patients were submitted to resection of the conal septum and, in three patients, enlargement of the IVC was necessary in its anterior portion. Six patients are being accompanied by Doppler echocardiography every six months without significant gradients being detected as of yet.
Anonymous insertion of the tricuspid valve at the IVC edge may interfere in preparing the tunnel, however there are some technical resources: 1 - freeing the papillary muscle and reinserting it in the tunnel wall [6] or 2 - the use of a flap constituted of the papillary muscle and the edge of the IVC; after preparing the tunnel, this flap is sutured into the wall of the tunnel [9]. The authors did not observe residual tricuspid insufficiency with these maneuvers.
Straddling of the tricuspid valve is an anatomic anomaly which is more difficult to repair at the time of preparing the LV-Ao tunnel and is associated with greater morbimortality rates [11].
In our series, neither anonymous insertion of the tricuspid valve nor straddling of the tricuspid valve was seen.
CONCLUSION
In conclusion, in this current work using the Lecompte operation we suggest that this technique may be employed in the anatomic correction of several types of anomalies of the A-V connection associated with IVC and obstruction of the pulmonary flow with a low incidence of morbimortality.
The Lecompte procedure, in this group of patients presents the following advantages:
· Surgical indication in younger patients;
· Low morbimortality rates;
· Long-term accompanying free of reoperations;
· It allows successful conversion of the Rastelli operation to the Lecompte procedure.
REFERENCES
1. Rastelli GC, McGoon DC, Wallace RB. Anatomic correction of transposition of great arteries with ventricular septal defect and subpulmonary stenosis. J Thorac Cardiovasc Surg. 1969;58(4):545-52.
2. Lecompte Y, Neveux JY, Leca F, Zannini L, Tu TV, Duboys Y, et al. Reconstruction of the pulmonary outflow tract without prosthetic conduit. J Thorac Cardiovasc Surg. 1982;84(5):727-33.
3. Marcelletti C, Mair DD, McGoon DC, Wallace RB, Danielson GK. The Rastelli operation for transposition of the great arteries: early and late results. J Thorac Cardiovasc Surg. 1976;72(3):427-34.
4. Moulton AL, de Leval MR, MaCartney FJ, Taylor JF, Stark J. Rastelli procedure for transposition of the great arteries, ventricular septal defect, and left ventriclar outflow tract obstruction: early and late results in 41 patients (1971 to 1978). Br Heart J. 1981;45(1):20-8.
5. Kirklin, JW, Barratt-Boyes BG. Complete transposition of the great arteries. In: Kirklin JW, Barratt-Boyes BG, eds. Cardiac surgery. New York:Wiley;1986. p.1129-37.
6. Lee JR, Lim HG, Kim YJ, Rho JR, Bae EJ, Noh CI, et al. Repair of transposition of the great arteries, ventricular septal defect and left ventricular outflow tract obstruction. Eur J Cardiothorac Surg. 2004;25(5):735-41.
7. Houyel L, Van Praagh R, Lacour-Gayet F, Serraf A, Petit J, Bruniaux J, et al. Transposition of the great arteries [S,D,L]: pathologic anatomy, diagnosis, and surgical management of a newly recognized complex. J Thorac Cardiovasc Surg. 1995;110(3):613-24.
8. Daskalopoulos DA, Edwards WD, Driscol DJ, Danielson GK, Puga FJ. Coronary artery compression with fatal myocardial ischemia: a rare complication of valved extracardiac conduits in children with congenital heart disease. J Thorac Cardiovasc Surg. 1983;85(4):546-51.
9. Borromée L, Lecompte Y, Batisse A, Lemoine G, Vouhé P, Sakata R, et al. Anatomic repair of anomalies of ventriculoarterial connection associated with ventricular septal defect. II. Clinical results in 50 patients with pulmonary outflow tract obstruction. J Thorac Cardiovasc Surg. 1988;95(1):96-102.
10. Jonas RA, Freed MD, Mayer JE Jr, Castaneda AR. Long-term follow-up of patients with synthetic right heart conduits. Circulation. 1985;72(3 pt 2):II77-83.
11. Kreutzer C, De Vive J, Oppido G, Kreutzer J, Gauvreau K, Freed M, et al. Twenty-five-year experience with Rastelli repair for transposition of the great arteries. J Thorac Cardiovasc Surg. 2000;120(2):211-23.
12. Homann M, Haehnel JC, Mendler N, Paek SU, Holper K, Meisner H, et al. Reconstruction of the RVOT with valved biological conduits: 25 years experience with allografts and xenografts. Eur J Cardiothorac Surg. 2000;17(6):624-30.
13. Razzouk AJ, Williams WG, Cleveland DC, Coles JG, Rebeyka IM, Trusler GA, et al. Surgical connections from ventricle to pulmonary artery. Comparison of four types of valved implants. Circulation. 1992;86(5 Suppl):II154-8.
14. Vouhé PR, Tamisier D, Leca F, Ouaknine R, Vernant F, Neveux JY. Transposition of great arteries, ventricular septal defect, and pulmonary outflow tract obstruction: Rastelli or Lecompte procedure? J Thorac Cardiovasc Surg. 1992;103(3):428-36.
15. Lecompte Y. Réparation á l'etage ventriculaire - the REV procedure: technique and clinical results. Cardiol Young. 1991;1(1):63-70.
16. Kim YJ, Song H, Lee JR, Rho JR, Suh KP. Lecompte procedure for complete transposition of the great arteries with ventricular septal defect and pulmonary stenosis. Ann Thorac Surg. 1994;57(4):876-9.
17. Metras D, Kreitmann B, Riberi A, Yao JG, El-Khoury E, Wernert F, et al. Extending the concept of the autograft for complete repair of transposition of the great arteries with ventricular septal defect and left ventricular outflow tract obstruction: a report of ten cases of a modified procedure. J Thorac Cardiovasc Surg. 1997;114(5):746-54.
18. Scavo VA Jr, Turrentine MW, Aufiero TX, Sun K, Binford R, Carlos G, Brown JW. Monocusp valve and transannular patch reconstruction of the right ventricular outflow tract: an experimental study. ASAIO J. 1998;44(5):M480-5.
19. Roughneen PT, DeLeon SY, Parvathaneni S, Cetta F, Eiden B, Vitulio DA. The pericardial membrane pulmonary monocusp: surgical technique and early results. J Card Surg. 1999;14(5):370-4.
20. Van Son JA, Sim EK. Lecompte operation with preservation of the pulmonary valve for anomalies of ventriculoarterial connection with ventricular septal defect and subpulmonary stenosis. Eur J Cardiothorac Surg. 1996;10(7):585-9.
21. Bando K, Danielson GK, Schaff HV, Mair DD, Julsrud PR, Puga FJ. Outcome of pulmonary and aortic homografts for right ventricular outflow tract reconstruction. J Thorac Cardiovasc Surg. 1995;109(3):509-18.
22. Maluf MA, Braile DM, Silva C, Catani R, Carvalho AC, Buffolo E. Reconstruction of the pulmonary valve and outflow tract with bicuspid prosthesis in tetralogy of Fallot. Ann Thorac Surg. 2000;70(6):1911-7.










 All scientific articles published at bjcvs.org are licensed under a Creative Commons license
All scientific articles published at bjcvs.org are licensed under a Creative Commons license