INTRODUCTION
Co-morbidity is common in patients with abdominal aortic aneurysms (AAA) [1]. The majority of AAA-patients have manifestations of cardiovascular disease such as myocardial infarction, stroke or hypertension, as well as pulmonary problems [2-4]. These co-morbidities require several medication-controlled treatments at the same time. The importance of medication in the prevention of secondary events is well known [5], but there is a risk of side effects or interaction of drugs in patients undergoing endovascular aneurysm repair (EVAR).
The reported incidence of complications after EVAR varies from 20% to 75% [6]. This morbidity is considerable and requires further investigation [6]. While in the earlier series complications could be attributed to suboptimal device construction, present stent-graft and delivery systems have improved and are fairly standardised [7]. With the use of improved equipment, the patient and doctor related risk factors for failure or complications are becoming more important determinants [6,8,9]. These factors have not been very well defined at the present time [6]. Various conditions may be expected to influence the occurrence of complications such as the learning curve, and anatomic characteristics of the aneurysm [10,11]. Also patient factors such as age, ASA-class and medication may influence the occurrence of complications and prolong hospital stay [2,6].
Until now, no research has been carried out on the influence of medication side effects or interactions on the occurrence of complications in AAA-patients with endovascular aneurysm repair.
METHOD
Subjects
Patients with endovascular abdominal aneurysm repair (EVAR) or attempted EVAR between April 1999 and November 2003 in two vascular surgical centres were included in the study. Experienced surgeons and interventional radiologists jointly performed implantation of stent-grafts. When necessary, adjuvant procedures such as the placement of extension devices of iliac limbs, coil embolisation of side branches, endarterectomy or patch-plasty of the common femoral artery were performed to obtain a satisfactory result. All patients were placed in the intensive care unit for monitoring during the first 4-20 hours after EVAR. On the first post-operative day, patients started with acetylsalicyl-acid (ASA) at a dosage of 80-100 mg per day as a standard medication. The follow-up protocol included contrast-enhanced CT-scanning on the first postoperative day and at 6, 12, 18, 24 months and annually thereafter.
Data assessment
Treatment results were assessed by clinical examination and by imaging studies of the vascular morphology and endograft during follow-up.In case of death, details of this event were recorded as they occurred.
Medication was assessed from medical records. To obtain the influence of medication on the occurrence of complications, we studied groups of drug as they were used by our patients, such as coumarin derivatives, b-blockers, calcium-antagonists, thiazide-diuretics and lipid lowering medication. Complications were also assessed from medical files and verified thereafter. They were classified into device related (such as rupture or endoleak type I or III) or non-device- related (such as endoleak type II or myocardial infarction) complications according to the SVS-ISCVS-score. Explicit probability of causes of complications was determined. All radiological investigations where therefore reviewed by a vascular surgeon and a radiologist and were judged whether technical satisfaction was obtained. The combination of duplex ultrasound-scan (DUS) and computed tomographic-angiography (CTA) allowed accurate identification of any endoleak source (type I-IV).
Covariates
The study variables included patient characteristics such as smoking, obesity, ASA-class, hypertension and co-morbidity according to the SVS-ISCVS risk score, as well as stentgraft type.
Data analysis
Chi-square analysis was used to compare the baseline characteristics of the subjects. Rates and relative risks were calculated by means of the number of complications in each patient-category. To quantify the correlation between clinical variables, medication, complications and mortality a logistic regression and Cox proportional hazards model was constructed. a of 0.05 was used to define statistical significance. All analyses were performed with The SAS system (version 8.00, SAS Institute, Cary, NC, USA).
RESULTS
The population consisted of 70 patients of which 66 were male. The mean age was 71.8 years (SD: 8.1, Range: 41-86) and the average aneurysm diameter was 62.5mm (SD: 10.3, Range: 50-110). The mean hospital stay was 4.1 days (SD: 4.9 Range: 0-31) and mean follow up 9.9 months (SD: 11.9, Range: 0-37). Eight patients (11%) were classified as ASA class I, 20 patients ASA class II (29%), 34 ASA class III (49%) and 8 ASA class IV (11%). Smoking was encountered in 28 patients (26%), hypertension in 36 (51%) and 11 patients were obese (16%). All operative procedures were planned for non-ruptured asymptomatic aneurysms. The mean duration of operation was 163 minutes (SD: 52.7 Range: 90-320) (Table 1).
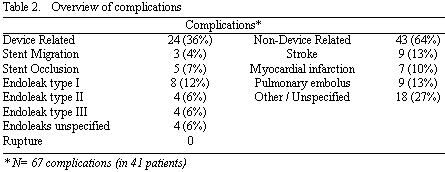
Overall, 41 patients (59%) experienced one or more complications (N=67) of which 24 were device-related and 43 non-device-related complications (Table 2). Most complications were in the early post-operative period. There were 20 endoleaks (29%) of which 8 were type I (11%), 4 endoleaks type II (6%), 4 endoleaks type III (6%), 0 type IV and 4 unspecified endoleaks (6%). No stent-graft infections or AAA-ruptures were encountered. Four patients died postoperatively (6%) of which two due to a malignancy after 2 months, one due to a pulmonary embolus at 3 months and one (1.4%) died within the 30 day period due to respiratory insufficiency. No cause of death was aneurysm-related in this study.
Table 3 shows an overview of the medication and number of complications. All patients (n=70) used thrombocyte aggregation inhibitors (ASA 80-100mg) by protocol. Other frequently used medication were
b-blockers (49%), RAS inhibitors (44%), coumarin-derivatives (43%). The in-hospital medication consisted of analgesics (36%), lipid-lowering drugs (23%), anti-emetics (29%) and calcium-antagonists (23%) respectively.
Patients who used analgesics during hospital stay (36%) showed more device related complications (P= 0.01) compared to patients who did not used this drug during hospital stay. Patients using anti-emetic drugs during hospital-stay (29%) showed more non-device-related complications (P= 0.036) compared to non-users. Patients who used coumarin-derivatives showed less non-device-related complications (P= 0.035) compared to non-users. The grade of severity of complications is presented in Table 4.
DISCUSSION
Presently, little is known about the influence of medication on the occurrence of complications after endovascular aneurysm repair (EVAR), whereas recognizing certain patterns in pathology and causes of complications is important [12,13] . As delivery and stent-graft systems have improved and are fairly standardized [6,14,15], risk profiles such as co-morbidity and medication are becoming more important factors for making evidence based decisions concerning elective endovascular therapy in patients with an aneurysm of the abdominal aorta (AAA) [6]. It is also important to detect whether in the post-operative period the risks for developing complications might be diminished. When more patient-related factors will be identified, appropriate alternatives in follow-up or medication may be developed. This study was designed to investigate whether additional medication is associated with any risk of complications in AAA-patients after EVAR.
The EVAR population consists of cardiovascular patients [16-18]. The preventive effect of thrombocyte-aggregation inhibitors in this population is already proven and therefore implemented in the EVAR-protocol [12,19-21]. Many cardiovascular patients are using coumarin-derivatives on a daily basis besides standard acetylsalicylic acid [3,12,19]. In our population the use of both thrombocyte aggregation inhibitors (by protocol) and coumarin-derivatives was quite common (e.g. 43%). This combination provides a protective effect against acute vascular events [5,20]. There are different opinions about the use of coumarin-derivatives next to thrombocyte-aggregation inhibitors after a device implant. Because of the anti-thrombotic effect of coumarin-derivatives [5] it was expected that more endoleaks could occur because of the occurence of a hyperthrombenemic state. This could not be confirmed in the present study. Patients using coumarin-derivatives in this study had less non-device-related complications than patients who did not.The use of analgesics during hospital stay was associated with significantly more device-related complications.
Patients using anti-emetic drugs during hospital-stay had more non-device-related complications. We have no explanation for these outcomes.
The mortality rate of 1.4% in the present study population is comparable to those of the EVAR-1 trial and the DREAM trial [22-24]. In these studies a mortality rate of 2.7% and 3% was recorded for elective EVAR. The initial survival advantage over open aneurysm repair, however, was not sustained after the first postoperative year. The main cause of late postoperative death in both trials was cardiovascular, confirming the impact of comorbidity in EVAR patients.
The EVAR-2 study was conducted in patients unfit for open aneurysm repair. It showed that EVAR is not a safe procedure in such high-risk patients [23]. It also raised concern about the medical treatment of these patients, fuelling the attention for co-morbidity and medication.
In conclusion, medication seems to be associated with the occurrence of complications following endovascular therapy of abdominal aortic aneurysms. Patients on coumarin-derivatives experienced fewer non-device-related complications. Patients who used anti-emetic drugs during hospital-stay showed a fourfold number of non-device-related complications. Patients using analgesics during hospital stay were associated with significantly more device-related complications.
REFERENCES
1. Faries PL, Brener BJ, Connelly TL, Katzen BT, Briggs VL, Burks JA Jr et al. A multicenter experience with the Talent endovascular graft for the treatment of abdominal aortic aneurysms. J Vasc Surg. 2002;35(6):1123-8.
2. Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35(5):1048-60.
3. Hansman MF, Neuzil D, Quigley TM, Hauptmann E, Fotoohi M, Robinson D et al. A comparison of 50 initial endoluminal endograft repairs for abdominal aortic aneurysm with 50 concurrent open repairs. Am J Surg. 2003;185(5):441-4.
4. Zarins CK, Wolf YG, Lee WA, Hill BB, Olcott IC, Harris EJ et al. Will endovascular repair replace open surgery for abdominal aortic aneurysm repair? Ann Surg. 2000;232(4):501-7.
5. Brouwer MA, van den Bergh PJ, Aengevaeren WR, Veen G, Luijten HE, Hertzberger DP et al. Aspirin plus coumarin versus aspirin alone in the prevention of reocclusion after fibrinolysis for acute myocardial infarction: results of the Antithrombotics in the Prevention of Reocclusion in Coronary Thrombolysis (APRICOT)-2 Trial. Circulation. 2002;106(6):659-65.
6. Cuypers P, Nevelsteen A, Buth J, Hamming J, Stockx L, Lacroix H et al. Complications in the endovascular repair of abdominal aortic aneurysms: a risk factor analysis. Eur J Vasc Endovasc Surg. 1999;18(3):245-52.
7. Riambau V, Laheij RJ, Garcia-Madrid C, Sanchez-Espin G. The association between co-morbidity and mortality after abdominal aortic aneurysm endografting in patients ineligible for elective open surgery. EUROSTAR Group. Eur J Vasc Endovasc Surg. 2001;22(3):265-70.
8. Conners MS 3rd, Tonnessen BH, Sternbergh WC 3rd, Carter G, Yoselevitz M, Money SR. Does ASA classification impact success rates of endovascular aneurysm repairs? Ann Vasc Surg. 2002;16(5):550-5.
9. Albertini JN, Branchereau A, Hopkinson B, Magnan PE, Bartoli JM, Whitaker SC et al. Mortality and morbidity following endovascular repair of abdominal aortic aneurysms: analysis of two single centers experiences. Eur J Vasc Endovasc Surg. 2001;22(5):429-35.
10. Arko FR, Fillis KA, Siedel SA, Johnson BL, Drake AR, Fogarty TJ et al. Intrasac flow velocities predict sealing of type II endoleaks after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2003;37(1):8-15.
11. Buth J, Laheij RJ. Early complications and endoleaks after endovascular abdominal aortic aneurysm repair: report of a multicenter study. J Vasc Surg. 2000;31(1 Pt 1):134-46.
12. Smout J, Stansby G. Current practice in the use of antiplatelet agents in the peri-operative period by UK vascular surgeons. Ann R Coll Surg Engl. 2003;85(2):97-101.
13. Blankensteijn JD, Lindenburg FP, van der Graaf Y, Eikelboom BC. Influence of study design on reported mortality and morbidity rates after abdominal aortic aneurysm repair. Br J Surg. 1998;85(12):1624-30.
14. Bernhard VM, Mitchell RS, Matsumura JS, Brewster DC, Decker M, Lamparello P et al. Ruptured abdominal aortic aneurysm after endovascular repair. J Vasc Surg. 2002;35(6):1155-62.
15. Parlani G, Verzini F, Zannetti S, De Rango P, Lenti M, Lupattelli L et al. Does gender influence outcome of AAA endoluminal repair? Eur J Vasc Endovasc Surg. 2003;26(1):69-73.
16. Criado FJ, Wilson EP, Fairman RM, Abul-Khoudoud O, Wellons E. Update on the Talent aortic stent-graft: a preliminary report from United States phase I and II trials. J Vasc Surg. 2001;33(2 Suppl):S146-9.
17. Chaikof EL, Lin PH, Brinkman WT, Dodson TF, Weiss VJ, Lumsden AB et al. Endovascular repair of abdominal aortic aneurysms: risk stratified outcomes. Ann Surg. 2002;235(6):833-41.
18. Virgillio CH, Romero L, Donayre C, Meek K, Lewis RJ, Lippmann M et al. Endovascular abdominal aortic aneurysm repair with general versus local anesthesia: a comparison of cardiopulmonary morbidity and mortality rates. J Vasc Surg. 2002;36(5):988-91.
19. Utsunomiya T, Krausz MM, Dunham B, Mannick JA, Allen PD, Shepro D et al. Maintenance of cardiodynamics with aspirin during abdominal aortic aneurysm ectomy (AAA). Ann Surg. 1981;194(5):602-8.
20. Kontogiorgis CA, Hadjipavlou-Litina DJ. Synthesis and biological evaluation of novel coumarin derivatives with a 7-azomethine linkage. Bioorg Med Chem Lett. 2004;14(3):611-4.
21. Weber MA. Calcium channel antagonists in the treatment of hypertension. Am J Cardiovasc Drugs. 2002;2(6):415-31.
22. Blankensteijn JD, de Jong SE, Prinssen M, van der Ham AC, Buth J, van Sterkenburg SM et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. Dutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group. N Eng J Med. 2005;352(23):2398-405.
23. EVAR Trial Participants. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 2005;365(9478):2187-92.
24. EVAR Trial Participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005; 365(9478):2179-86.


 All scientific articles published at bjcvs.org are licensed under a Creative Commons license
All scientific articles published at bjcvs.org are licensed under a Creative Commons license