Objective: Permanent cardiac pacing improves survival of children with congenital or acquired bradycardia, although mortality after pacing remains relatively high. The aim of this study was to evaluate the long-term outcomes of children who undergo permanent endocardial cardiac pacing via the femoral vein, including the identification of associated risk factors for mortality.
Method: From 1981 to 2000, 99 patients ranging in ages from one day to 13 years (4.1 ± 3.6 and median = 3 years) underwent permanent transfemoral pacemaker implantation due to congenital (39.4%), postsurgical (54.5%), or non-surgically acquired bradycardia (6.1%).
Results: By the end of 7.1 ± 5.3 years (708.3 patient-years) of prospective follow-up, 18 (18.2%) patients had died. The actuarial survival rates were 85%, 79.5%, and 74.2%, at 5, 10, and 15 years, respectively. Independent predictors of mortality identified by Cox proportional hazards analysis were younger age at implantation (p = 0.028), the presence of untreated cardiac anomalies or intracardiac prostheses (p = 0.0001), and radiographic evidence of cardiomegaly (p = 0.035).
Conclusions: Permanent endocardial pacing via the femoral vein presented survival expectance comparable to other techniques with a low rate of pacing complications. Long-term survival was limited by lower ages and cardiac dilatation at the time of implantation as well as by the presence of untreated cardiac defects or valve prostheses.
Objetivo: A estimulação cardíaca permanente melhora a sobrevida de crianças com bradicardia congênita ou adquirida, embora a mortalidade após o implante de marcapasso permaneça relativamente alta. O objetivo deste estudo foi avaliar os resultados em longo prazo de crianças submetidas a implante de marcapasso endocárdico pela veia femoral, incluindo a identificação de fatores de risco associados à mortalidade.
Método: De 1981 a 2000, 99 pacientes variando em idade de um dia a 13 anos (4,1 ± 3,6 com mediana = 3 anos) foram submetidos a implante de marcapasso permanente pela via femoral devido a bradicardia de origem congênita (39,4%), pós-cirúrgica (54,5%) ou adquirida não cirurgicamente (6,1%).
Resultados: Ao final de 7,1 ± 5,3 anos (708,3 pacientes-anos) de seguimento, 18 (18,2%) pacientes haviam morrido. A sobrevida atuarial foi de 85%, 79,5%, e 74,2%, aos cinco, 10, e 15 anos, respectivamente. Os fatores independentes de mortalidade identificados pela análise proporcional de Cox foram: menor idade ao implante (p = 0,028), presença de anomalias cardíacas não corrigidas ou presença de próteses intracardíacas (p = 0,0001) e evidências radiográficas de cardiomegalia (p = 0,035).
Conclusões: A estimulação cardíaca endocárdica permanente pela via femoral apresenta expectativa de sobrevida comparável a outras técnicas, com baixas taxas de complicações devidas ao implante de marcapasso. A sobrevida em longo prazo foi limitada pela menor idade e dilatação cardíaca no momento do implante, assim como pela presença de defeitos cardíacos sem correção ou de próteses valvares.
INTRODUCTION
Permanent cardiac pacing reduces mortality of children with congenital or acquired bradycardia, although in respect to the pediatric population, the total mortality rate remains high with a five-year survival rate of from 74% to 78% [1-4]. The commonest cause of death is subjacent disease, while mortality related to pacemaker implantation complications is reported in from 2% to 4% of the cases [5].
Epimyocardial implantations by transthoracic or subxiphoid access are preferred in children with less than 15 kg. However, lower rates of complications have been reported with endocardial electrodes. The transvenous access via the femoral vein was proposed for pediatric patients of all age ranges from newborns to teenagers, with good pacing performance and sensitivity over the long-term [6,7].
The aim of this study was to evaluate the long-term outcomes of children who underwent permanent endocardial cardiac pacing by femoral access, in order to identify risk factors associated with mortality.
METHOD
Studied population
From November 1981 to November 2000, 264 patients with ages of up to 18 years old were submitted to first permanent pacemaker implantation in the Heart Institute of the Medical School, São Paulo University. Epimyocardial pacemakers were implanted in 35 patients (13.3%) and endocardial cardiac pacing via the subclavian, jugular or femoral veins in 229 patients (86.7%). In this institution, transfemoral endocardial access is the preferred choice for pre-adolescents, with the classical approach by the subclavian vein used with teenagers. The epimyocardial access has been utilized in low-weight neonates or always when a shunt is detected between right and left chambers, when there is risk of systemic thromboembolism.
This study included 99 children who underwent transfemoral electrode implantation, prospectively followed up for up to 20.7 years.
For the first implantation, the age varied from one day to thirteen years (mean age = 4.1 ± 3.6 years, median age = 3 years). Fifty-six patients were female and 43 patients were male.
Postoperative bradycardia was the main reason for implantation (54.5%), followed by congenital rhythm disorders (39.4%) and non-surgical acquired bradycardia (6.1%)
History of syncope was present in 11.1% and giddiness or pre-syncope was reported by 10.1% of patients. At the first implantation, no sign or symptom of heart insufficiency was identified in 31.3% of the patients, in 36.4% of the patients the symptoms were slight and in 32.3% of them the symptoms were moderate or significant.
Total atrioventricular block (TAVB) was the main electrocardiographic finding in 78.7% of the cases. Intermittent TAVB was seen in 5.1% of the cases, 2nd degree type II AVB in 5.1%, sinus-node disease in 8.1% and congenital long QT syndrome in 3.0% with permanent pacing recommended for the other patients.
The size of the heart area, observed by chest radiograph, was considered normal or slightly increased in 35.4% and moderately or severely increased in 64.6% of the children.
Only 33 children (33.3%) did not present any intracardiac defect before the first implantation. Seven patients presented with non-detected intracardiac defects at the moment of the first implantation, as echocardiography was not available for all patients at the beginning of this period. These defects were discovered and corrected during the follow-up or observed during the autopsy
The surgical repair of intracardiac defects had been previously performed in 59 (59.6%) children. In 52 patients, the repair was considered complete, although AV discordance persisted in four and extra-cardiac grafts were necessary in three patients. In seven patients, serious hemodynamic problems persisted after the surgical repair: five children were submitted to palliative procedures or the repair was considered incomplete and two patients received mechanical valvar prostheses. One child was submitted to heart transplantation.
Eight patients presented with non-cardiac problems: Down syndrome (five), epilepsy (one), autism (one) and strokes with hemiparesis (one).
Implantation technique
Ventricular pacemakers were implanted via the saphenous or internal femoral veins in 88.9% of the cases, atrioventricular pacemakers in 7.1% and atrial pacemakers in 4.0% of the children. Pulse generators were implanted in the supra-inguinal area in subcutaneous or subaponeurotic positions on the left side in 91.9% of the cases and on the right side in 8.9%. The implantation technique has previously been described by Costa et al. [6,7].
Predictors of long-term mortality
The following risk factors of death were studied: age at implantation, gender, cause of bradycardia, degree of cardiac insufficiency, size of the cardiac area by chest radiograph, advanced AVB and the presence of hemodynamic disorders.
The causes of bradycardia were identified as surgically or non-surgically induced, cardiac insufficiency as asymptomatic/discreetly symptomatic against moderate/seriously symptomatic and AVB, as 2nd/ 3rd degree versus AV conduction 1:1. The size of the heart area was considered as normal/slightly increased or moderate/severely increased and the hemodynamic disorders included patients with non-corrected intracardiac defects or with valvar prosthesis versus patients without intracardiac defects or who underwent complete repairs.
Statistic analysis
Expected mortality over time was determined by the Kaplan-Meier's non-parametric method. Exploratory analysis of risk factors, including analysis of correlation, preceded the multivariable analysis of the outcomes. The Cox proportional-hazards model was employed in the multivariable analysis and the differences among the event frequencies over time according to the independent variable mortality predictors were compared using the Log-Rank test.
All data were analyzed using the Statistical Package for Social Sciences (SPSS) software, with p-values < 0.05 being considered significant.
RESULTS
The mean follow-up was 7.1 ± 5.3 years, with a variation of seven days to 20.7 years (708.3 patient-years). Of the six patients lost during the follow-up, the first loss occurred in six months and the last in 5.3 years after the pacemaker implantation (mean: 2.7 ± l.4 years).
At the first implantation, the initial atrial stimulation varied from 0.3 to 2.0 V (mean = 0.6 ± 0.5 V) and the initial ventricular stimulation varied from 0.1 to 1.3 V (mean = 0.6 ± 0.2 V).
Reoperations performed to maintain the pacing system were necessary in 107 situations due to: the necessity of battery exchange or the growth of the child (72), infection (12), pacing defect (10) and other causes (13). Seven patients were submitted to a change in the pacing mode because of congestive heart failure: to an atrioventricular mode (two), to an atrio-biventricular mode (three) and to an atrio-bifocal mode in the right ventricle (two)
After the initial pacemaker implantation, one or more open surgeries (seven) or heart transplantation (one) were performed in seven children: to complete a previous surgical repair; to correct a defect not previously detected or due to the late onset heart disease. The cardiac pacing was permanently discontinued by heart transplantation in one and after recovery of the AV-conduction in two children.
Eighteen patients (18.2%) died during the follow-up period. The earliest death occurred seven days after pacemaker implantation and the last after 13.4 years. The causes of death were terminal heart failure (five): infection (five); sudden death (two); pulmonary bleeding (one) and anesthesia complications (one). In four patients, the cause of death remains unknown.
The expected survival rates over five, ten and fifteen years were 85.0%, 79.5% and 74.2%, respectively (Figure 1).
An exploratory analysis of the preoperative variables excluded gender, cause of the bradycardia and advanced AVB as risk factors.
The multivariable analysis identified hemodynamic disturbances, moderately or severely increased heart area and low ages at implantation as independent risk factors for mortality.
The influence of hemodynamic disturbances due to persistent intracardiac defects or valvar prostheses as risk factors can be seen in Figure 2. The mortality rate was expressively higher in patients with intracardiac defects or valve prostheses than in patients without associated cardiac injuries.
Patients with moderately or severely increased heart area seen by chest radiography had a worse prognosis than patients with normal or slightly increased areas (Figure 3).
Children older than three years of age at the moment of the first implantation had a more favorable long-term survival than under three-year-old patients (Figure 4).
COMMENTS
The two main indications for pacemaker implantation in children are congenital and postoperative conduction blocks. The great number of children with congenital atrioventricular blocks who also have intracardiac defects, frequently makes the differentiation of these patients from patients suffering from post-surgical blocks difficult. Data of four retrospective studies involving 650 children with permanent pacemakers revealed post-operative bradycardia in from 40% to 69% of the cases, congenital AVB in from 19% to 42%, and structural cardiac defects in from 44% to 84% [1-4].
Although data on long-term outcomes of all types of pediatric pacing are still very limited, the literature shows a total mortality rate that varies from 4.l% to 18.9% over the mid-term with follow-ups of from 2.4 to 11.9 years [1-4]. Subjacent heart diseases are the main cause of death in patients who require pacemakers; but, complications related to the implantation can be a cause of death and occur in up to 4% of patients [5].
The hemodynamic impact of non-corrected structural cardiac defects and of mechanic valvar prostheses on the mortality rate observed in the current study can also be expected in patients without pacemakers. Whilst the children who were submitted to complete repair of the congenital heart defects present high long-term survival rates, in spite of the use of intracardiac patches, extracardiac tubes or present with persistence of the AV discordance, the use of valvar prostheses or the presence of non-corrected defects has been related to high mortality rates [8-10]. It is important to consider though, systemic thromboembolism as a possible cause of death, since some children have non-repaired or residual septal defects, even though no cases of thromboembolism were clinically detected or found during autopsies in this series. If non-corrected defects were diagnosed during the pacemaker implantation procedure, an epimyocardial approach may have been a better option in these cases. But, it is impossible to know if the mortality rate of these patients would have been different with epimyocardial pacemakers.
The influence of the age at the first implantation on the mortality rate encountered in the current study has been reported in other series. The mortality rate of children with congenital TAVB (CTAVB), diagnosed in the uterus or in the new-born baby, is reported at 19% [11] and after the neonatal period it seems to be lower, varying from 5% to 10% [12]. Studying children with CTAVB diagnosed with ages of from three months to fifteen years, Eronen [13] observed a mortality rate of 5% after a mean follow-up period of 22 years. Serwer & Mericle [14] found another correlation between time of implantation and mortality rate: the majority of deaths occurred during the first six months after the first implantation while from six months to 16 years after the implantation the survival rate decreased only 16%. As no case of death related to the operative technique occurred in the present series, the more favorable long-term survival of over three-year-old children at the first implantation may simply be explained by the fact that, the more severe the disease is, the earlier the child needs to be operated on. On the other hand, children who underwent pacemaker implantation at an older age had already been submitted to a type of natural selection.
Cardiomegaly seen by chest radiography, the third risk factor identified in this study, was correlated to poor survival rates in other series, although a moderately enlarged left ventricle with normal wall tension and increased systolic function is a common observation during the first two decades of life in patients with CTAVB and can be reverted through cardiac pacing [15,16]. The development of dilated cardiomyopathy after pacemaker implantation has been described in 6% at 7% of patients with CTAVB [13,17,18]. The precise etiology of this late onset cardiomyopathy is unknown, but it seems to be related to the autoimmune process [19].
No influence of gender, etiology, presence of AVB and cardiac insufficiency were detected in respect to mortality. A considerable number of children with CTAVB also presented with intracardiac defects, which cause congenital bradycardia and a very similar physiopathology is often seen in the postoperative period. Additionally, many patients with moderate/severe symptomatic cardiac insufficiency also had a moderate/serious increase in cardiac area, which could explain the exclusion of cardiac insufficiency as a risk factor during the multivariable statistic analysis.
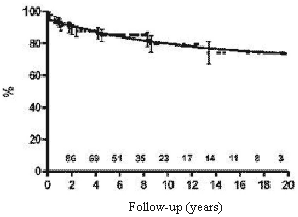
Fig. 1 - Calculation using the Kaplan-Meier method of the probability of survival of children submitted to permanent cardiac pacing by the transfemoral-endocardial approach. Numbers indicate the patients at risk at any particular moment
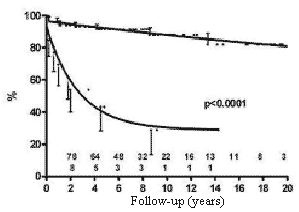
Fig. 2 - Influence of the presence of residual defects or of valve prostheses on the probability of survival of children submitted to permanent cardiac pacing by the transfemoral-endocardial approach. Circles = palliative corrections / total corrections with residual defects or valve prostheses; Squares = total corrections
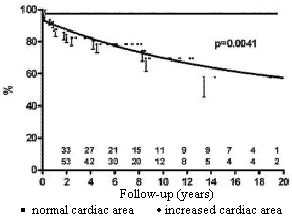
Fig. 3 - Influence of the size of the cardiac area in the preoperative period on the probability of survival of children submitted to permanent cardiac pacing by the transfemoral-endocardial approach. Circles = moderate / severely increased cardiac area; squares = normal / slightly increased cardiac area
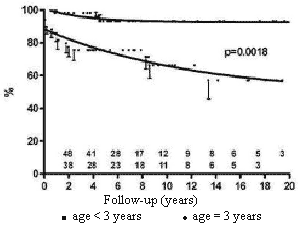
Fig. 4 - Influence of the age at time of first implantation on the probability of survival of children submitted to permanent cardiac pacing by the transfemoral-endocardial approach. Circles = age < 3 years; squares = age = 3 years
CONCLUSIONS
Permanent endocardial pacemaker implantation via the femoral vein in children with congenital or acquired bradycardia over a follow-up period of up to 20.7 years, gave survival rates comparable to other published series. Statistical analysis identified preoperative cardiomegaly, lower ages at the first implantation and the persistence of structural defects or valvar prostheses after pacemaker implantation as independent risk factors for mortality in this group of patients. The present data also suggest that patients with ongoing cardiomegaly and patients who underwent palliative or incomplete repair or who have valvar prosthesis present with a necessity of special attention.
BIBLIOGRAPHIC REFERENCES
1. Rao V, Williams WG, Hamilton RH, Williams MG, Goldman BS, Gow RM. Trends in pediatric cardiac pacing. Can J Cardiol. 1995;11(11):993-9.
2. Sachweh JS, Vazquez-Jimenez JF, Schöndube FA, Daebritz SH, Dorge H, Muhler EG et al. Twenty years experience with pediatric pacing: epicardial and transvenous stimulation. Eur J Cardiothorac Surg. 2000;17(4):455-61.
3. Cohen MI, Bush DM, Vetter VL, Tanel RE, Wieand TS, Gaynor JW et al. Permanent epicardial pacing in pediatric patients: seventeen years of experience and 1200 outpatient visits. Circulation. 2001;103(21):2585-90.
4. Thomson JD, Blackburn ME, Van Doorn C, Nicholls A, Watterson KG. Pacing activity, patient and lead survival over 20 years of permanent epicardial pacing in children. Ann Thorac Surg. 2004;77(4):1366-70.
5. Esperer HD, Singer H, Riede FT, Blum U, Mahmoud FO, Weniger J. Permanent epicardial and transvenous single- and dual chamber cardiac pacing in children. Thorac Cardiovasc Surg. 1993;41(1):21-7.
6. Costa R, Barbosa LCV, Moreira LFP, Martinelli Filho M, Fernandes PMP, Stolf NAG, et al. Marcapasso endocárdico definitivo na primeira década de vida. Rev Bras Cir Cardiovasc. 1986;1(2):15-9.
7. Costa R, Martinelli Filho M, Tamaki WT, Crevelari ES, Nishioka SD, Moreira LF et al. Transfemoral pediatric permanent pacing: long-term results. Pacing Clin Electrophysiol. 2003;26(1 Pt 2):487-91.
8. Driscoll DJ, Offord KP, Feldt RH, Schaff HV, Puga FJ, Danielson GK. Five- to fifteen-year follow-up after Fontan operation. Circulation. 1992; 85(2):469-96.
9. Gilljam T, Eriksson BO, Solymar L, Jonsson M. Status of survivors after atrial redirection for transposition of the great arteries: a complete long-term follow-up. Acta Paediatr. 1996;85(2):832-7.
10. Spevak PJ, Freed MD, Castaneda AR, Norwood WI, Pollack P. Valve replacement in children less than 5 years of age. J Am Coll Cardiol. 1986;8(4):901-8.
11. Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998; 31(7):1658-66.
12. Michaelsson M, Jonzon A, Riesenfeld T. Isolated complete atrioventricular block in adult life: a prospective study. Circulation. 1995;92(3):442-9.
13. Eronen M. Long-term outcome of children with complete heart block diagnosed after the newborn period. Pediatr Cardiol. 2001;22(2):133-7.
14. Serwer GA, Mericle JM. Evaluation of pacemaker pulse generator and patient longevity in patients aged 1 day to 20 years. Am J Cardiol. 1987;59(8):824-7.
15. Kertesz NJ, Friedman RA, Colan SD, Walsh EP, Gajarski RJ, Gray PS et al. Left ventricular mechanics and geometry in patients with congenital complete atrioventricular block. Circulation. 1997;96(10):3430-5.
16. Breur JM, Udink Ten Cate FE, Kapusta L, Cohen MI, Crosson JE, Boramanand N et al. Pacemaker therapy in isolated congenital complete atrioventricular block. Pacing Clin Electrophysiol. 2002;25(12):1685-91.
17. Eronen M, Siren MK, Ekblad H, Tikanoja T, Julkunen H, Paavilainen T. Short- and long-term outcome of children with congenital complete heart block diagnosed in utero or as a newborn. Pediatrics. 2000;106(1 Pt 1):86-91.
18. Udink ten Cate FE, Breur JM, Cohen MI, Boramanand N, Kapusta L, Crosson JE et al. Dilated cardiomyopathy in isolated congenital complete atrioventricular block: early and long-term risk in children. J Am Coll Cardiol. 2001;37(4):1129-34.
19. Taylor-Albert E, Reichlin M, Toews WH, Overholt ED, Lee LA. Delayed dilated cardiomyopathy as a manifestation of neonatal lupus: Case reports, autoantibody analysis, and management. Pediatrics. 1997;99(5):733-5.




 All scientific articles published at bjcvs.org are licensed under a Creative Commons license
All scientific articles published at bjcvs.org are licensed under a Creative Commons license